| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected and Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2023-02-21 17:15:11 UTC |
|---|
| HMDB ID | HMDB0000807 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 3-Phosphoglyceric acid |
|---|
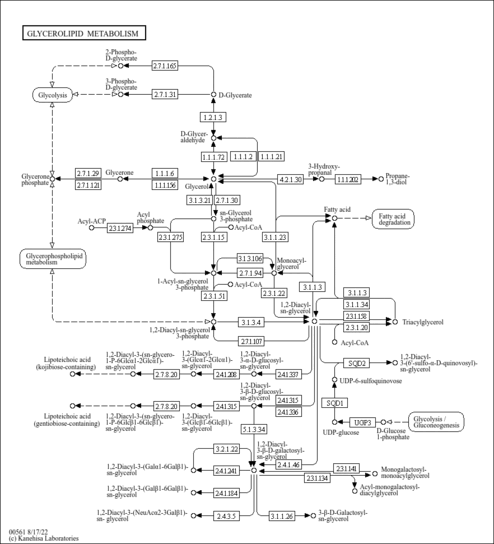
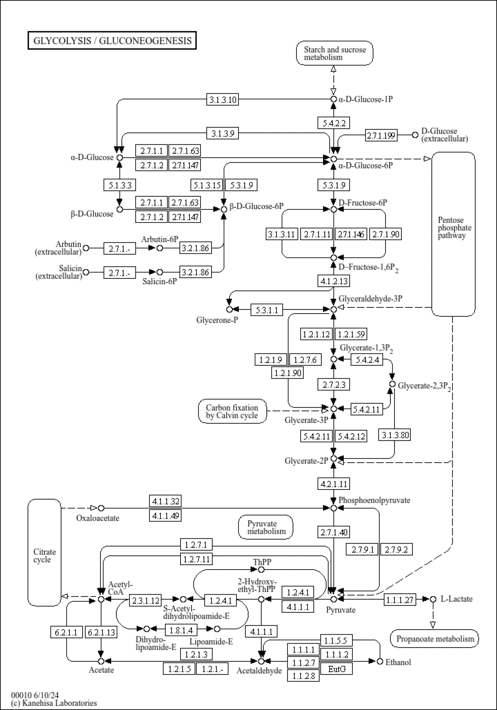
| Description | 3-Phosphoglyceric acid, also known as 3PG, belongs to the class of organic compounds known as sugar acids and derivatives. Sugar acids and derivatives are compounds containing a saccharide unit which bears a carboxylic acid group. 3PG is the conjugate acid of glycerate 3-phosphate (GP or G3P). It is a solid that is soluble in water. 3-Phosphoglyceric acid exists in all living species, ranging from bacteria to humans. The glycerate is a biochemically significant metabolic intermediate in both glycolysis and the Calvin cycle. This is the first compound formed during the C3 or Calvin cycle. Glycerate 3-phosphate is also a precursor for serine, which, in turn, can create cysteine and glycine through the homocysteine cycle. Within humans, 3-phosphoglyceric acid participates in a number of enzymatic reactions. In particular, 3-phosphoglyceric acid can be biosynthesized from glyceric acid 1,3-biphosphate, which is mediated by the enzyme phosphoglycerate kinase 1. In addition, 3PG can be converted into 2-phospho-D-glyceric acid, which is catalyzed by the enzyme phosphoglycerate mutase 2. 3-phosphoglyceric acid is involved in the Warburg effect (aerobic glycolysis), a metabolic shift that is a hallmark of cancer (PMID: 29362480 ). |
|---|
| Structure | InChI=1S/C3H7O7P/c4-2(3(5)6)1-10-11(7,8)9/h2,4H,1H2,(H,5,6)(H2,7,8,9) |
|---|
| Synonyms | | Value | Source |
|---|
| 3-(Dihydrogen phosphate)glycerate | ChEBI | | 3-(Dihydrogen phosphate)glyceric acid | ChEBI | | 3-Phosphoglycerate | ChEBI | | DL-Glycerate 3-phosphate | ChEBI | | Glycerate 3-phosphate | ChEBI | | Glycerate 3-phosphates | ChEBI | | Glyceric acid 3-phosphate | ChEBI | | 3-(Dihydrogen phosphoric acid)glyceric acid | Generator | | DL-Glyceric acid 3-phosphoric acid | Generator | | Glyceric acid 3-phosphoric acid | Generator | | Glyceric acid 3-phosphates | Generator | | 3-Glycerophosphorate | HMDB | | 3-Glycerophosphoric acid | HMDB | | 3-p-D-Glycerate | HMDB | | 3-p-Glycerate | HMDB | | 3-PG | HMDB | | 3-PGA | HMDB | | 3-Phospho-(R)-glycerate | HMDB | | 3-Phospho-D-glycerate | HMDB | | 3-Phospho-glycerate | HMDB | | 3-Phospho-glyceric acid | HMDB | | D-(-)-3-Phosphoglyceric acid | HMDB | | D-Glycerate 3-phosphate | HMDB | | G3P | HMDB | | Glycerate-3-p | HMDB | | Phosphoglycerate | HMDB | | 3-Phosphoglycerate, trisodium salt | HMDB | | 3-Phosphoglycerate, (R)-isomer | HMDB | | 3-Phosphoglycerate, monosodium salt | HMDB |
|
|---|
| Chemical Formula | C3H7O7P |
|---|
| Average Molecular Weight | 186.0572 |
|---|
| Monoisotopic Molecular Weight | 185.99293909 |
|---|
| IUPAC Name | 2-hydroxy-3-(phosphonooxy)propanoic acid |
|---|
| Traditional Name | phosphoglycerate |
|---|
| CAS Registry Number | 820-11-1 |
|---|
| SMILES | OC(COP(O)(O)=O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C3H7O7P/c4-2(3(5)6)1-10-11(7,8)9/h2,4H,1H2,(H,5,6)(H2,7,8,9) |
|---|
| InChI Key | OSJPPGNTCRNQQC-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as sugar acids and derivatives. Sugar acids and derivatives are compounds containing a saccharide unit which bears a carboxylic acid group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Sugar acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Glyceric_acid
- Monoalkyl phosphate
- Alpha-hydroxy acid
- Hydroxy acid
- Monosaccharide
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Alkyl phosphate
- Secondary alcohol
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Carboxylic acid
- Alcohol
- Carbonyl group
- Organic oxide
- Hydrocarbon derivative
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | Naturally occurring processBiological processBiochemical pathwayMetabolic pathwayDisease pathway- Dimethylglycine Dehydrogenase Deficiency (PathBank: SMP0000242)
- Dihydropyrimidine Dehydrogenase Deficiency (DHPD) (PathBank: SMP0000179)
- Sarcosinemia (PathBank: SMP0000244)
- Glycerol Kinase Deficiency (PathBank: SMP0000187)
- Glycogen Storage Disease Type 1A (GSD1A) or Von Gierke Disease (PathBank: SMP0000374)
- Non-Ketotic Hyperglycinemia (PathBank: SMP0000223)
- Hyperglycinemia, Non-Ketotic (PathBank: SMP0000485)
- D-Glyceric Acidura (PathBank: SMP0000529)
- Glycogenosis, Type VII. Tarui Disease (PathBank: SMP0000531)
- Glycogen Synthetase Deficiency (PathBank: SMP0000552)
- Glycogenosis, Type III. Cori Disease, Debrancher Glycogenosis (PathBank: SMP0000553)
- Glycogenosis, Type IV. Amylopectinosis, Anderson Disease (PathBank: SMP0000554)
- Glycogenosis, Type VI. Hers Disease (PathBank: SMP0000555)
- Mucopolysaccharidosis VI. Sly Syndrome (PathBank: SMP0000556)
- Sucrase-Isomaltase Deficiency (PathBank: SMP0000557)
- Phosphoenolpyruvate Carboxykinase Deficiency 1 (PEPCK1) (PathBank: SMP0000560)
- Fructose-1,6-diphosphatase Deficiency (PathBank: SMP0000562)
- Triosephosphate Isomerase (PathBank: SMP0000563)
- Fanconi-Bickel Syndrome (PathBank: SMP0000572)
- Glycogenosis, Type IB (PathBank: SMP0000573)
- Glycogenosis, Type IC (PathBank: SMP0000574)
- Glycogenosis, Type IA. Von Gierke Disease (PathBank: SMP0000581)
- 3-Phosphoglycerate Dehydrogenase Deficiency (PathBank: SMP0000721)
- Glycogenosis, Type IB (HMDB: HMDB0000807)
- Familial lipoprotein lipase deficiency (PathBank: SMP0120595)
|
|---|
| Role | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Experimental Collision Cross Sections |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| 3-Phosphoglyceric acid,1TMS,isomer #1 | C[Si](C)(C)OC(COP(=O)(O)O)C(=O)O | 1733.1 | Semi standard non polar | 33892256 | | 3-Phosphoglyceric acid,1TMS,isomer #2 | C[Si](C)(C)OC(=O)C(O)COP(=O)(O)O | 1699.7 | Semi standard non polar | 33892256 | | 3-Phosphoglyceric acid,1TMS,isomer #3 | C[Si](C)(C)OP(=O)(O)OCC(O)C(=O)O | 1720.8 | Semi standard non polar | 33892256 | | 3-Phosphoglyceric acid,2TMS,isomer #1 | C[Si](C)(C)OC(=O)C(COP(=O)(O)O)O[Si](C)(C)C | 1738.7 | Semi standard non polar | 33892256 | | 3-Phosphoglyceric acid,2TMS,isomer #2 | C[Si](C)(C)OC(COP(=O)(O)O[Si](C)(C)C)C(=O)O | 1773.9 | Semi standard non polar | 33892256 | | 3-Phosphoglyceric acid,2TMS,isomer #3 | C[Si](C)(C)OC(=O)C(O)COP(=O)(O)O[Si](C)(C)C | 1715.2 | Semi standard non polar | 33892256 | | 3-Phosphoglyceric acid,2TMS,isomer #4 | C[Si](C)(C)OP(=O)(OCC(O)C(=O)O)O[Si](C)(C)C | 1764.3 | Semi standard non polar | 33892256 | | 3-Phosphoglyceric acid,3TMS,isomer #1 | C[Si](C)(C)OC(=O)C(COP(=O)(O)O[Si](C)(C)C)O[Si](C)(C)C | 1778.9 | Semi standard non polar | 33892256 | | 3-Phosphoglyceric acid,3TMS,isomer #1 | C[Si](C)(C)OC(=O)C(COP(=O)(O)O[Si](C)(C)C)O[Si](C)(C)C | 1756.7 | Standard non polar | 33892256 | | 3-Phosphoglyceric acid,3TMS,isomer #1 | C[Si](C)(C)OC(=O)C(COP(=O)(O)O[Si](C)(C)C)O[Si](C)(C)C | 2134.9 | Standard polar | 33892256 | | 3-Phosphoglyceric acid,3TMS,isomer #2 | C[Si](C)(C)OC(COP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)C(=O)O | 1803.7 | Semi standard non polar | 33892256 | | 3-Phosphoglyceric acid,3TMS,isomer #2 | C[Si](C)(C)OC(COP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)C(=O)O | 1791.5 | Standard non polar | 33892256 | | 3-Phosphoglyceric acid,3TMS,isomer #2 | C[Si](C)(C)OC(COP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)C(=O)O | 2004.4 | Standard polar | 33892256 | | 3-Phosphoglyceric acid,3TMS,isomer #3 | C[Si](C)(C)OC(=O)C(O)COP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 1754.1 | Semi standard non polar | 33892256 | | 3-Phosphoglyceric acid,3TMS,isomer #3 | C[Si](C)(C)OC(=O)C(O)COP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 1746.7 | Standard non polar | 33892256 | | 3-Phosphoglyceric acid,3TMS,isomer #3 | C[Si](C)(C)OC(=O)C(O)COP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 1947.9 | Standard polar | 33892256 | | 3-Phosphoglyceric acid,4TMS,isomer #1 | C[Si](C)(C)OC(=O)C(COP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[Si](C)(C)C | 1833.3 | Semi standard non polar | 33892256 | | 3-Phosphoglyceric acid,4TMS,isomer #1 | C[Si](C)(C)OC(=O)C(COP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[Si](C)(C)C | 1819.9 | Standard non polar | 33892256 | | 3-Phosphoglyceric acid,4TMS,isomer #1 | C[Si](C)(C)OC(=O)C(COP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[Si](C)(C)C | 1902.5 | Standard polar | 33892256 | | 3-Phosphoglyceric acid,1TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(COP(=O)(O)O)C(=O)O | 1959.6 | Semi standard non polar | 33892256 | | 3-Phosphoglyceric acid,1TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OC(=O)C(O)COP(=O)(O)O | 1927.5 | Semi standard non polar | 33892256 | | 3-Phosphoglyceric acid,1TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OP(=O)(O)OCC(O)C(=O)O | 1953.2 | Semi standard non polar | 33892256 | | 3-Phosphoglyceric acid,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)C(COP(=O)(O)O)O[Si](C)(C)C(C)(C)C | 2126.7 | Semi standard non polar | 33892256 | | 3-Phosphoglyceric acid,2TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OC(COP(=O)(O)O[Si](C)(C)C(C)(C)C)C(=O)O | 2153.1 | Semi standard non polar | 33892256 | | 3-Phosphoglyceric acid,2TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC(=O)C(O)COP(=O)(O)O[Si](C)(C)C(C)(C)C | 2124.7 | Semi standard non polar | 33892256 | | 3-Phosphoglyceric acid,2TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)OP(=O)(OCC(O)C(=O)O)O[Si](C)(C)C(C)(C)C | 2174.8 | Semi standard non polar | 33892256 | | 3-Phosphoglyceric acid,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)C(COP(=O)(O)O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 2349.8 | Semi standard non polar | 33892256 | | 3-Phosphoglyceric acid,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)C(COP(=O)(O)O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 2389.7 | Standard non polar | 33892256 | | 3-Phosphoglyceric acid,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)C(COP(=O)(O)O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 2438.1 | Standard polar | 33892256 | | 3-Phosphoglyceric acid,3TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OC(COP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)C(=O)O | 2376.2 | Semi standard non polar | 33892256 | | 3-Phosphoglyceric acid,3TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OC(COP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)C(=O)O | 2347.6 | Standard non polar | 33892256 | | 3-Phosphoglyceric acid,3TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OC(COP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)C(=O)O | 2335.6 | Standard polar | 33892256 | | 3-Phosphoglyceric acid,3TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC(=O)C(O)COP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 2366.5 | Semi standard non polar | 33892256 | | 3-Phosphoglyceric acid,3TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC(=O)C(O)COP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 2367.3 | Standard non polar | 33892256 | | 3-Phosphoglyceric acid,3TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC(=O)C(O)COP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 2293.3 | Standard polar | 33892256 | | 3-Phosphoglyceric acid,4TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)C(COP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 2587.9 | Semi standard non polar | 33892256 | | 3-Phosphoglyceric acid,4TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)C(COP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 2530.9 | Standard non polar | 33892256 | | 3-Phosphoglyceric acid,4TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)C(COP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 2336.2 | Standard polar | 33892256 |
|
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental GC-MS | GC-MS Spectrum - 3-Phosphoglyceric acid GC-EI-TOF (Non-derivatized) | splash10-0002-0974000000-41479250991be9cc1e07 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - 3-Phosphoglyceric acid GC-EI-TOF (Non-derivatized) | splash10-0002-0974000000-41479250991be9cc1e07 | 2018-05-18 | HMDB team, MONA, MassBank | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 3-Phosphoglyceric acid GC-MS (Non-derivatized) - 70eV, Positive | splash10-0002-9200000000-2d6f0050126bbc72d771 | 2016-09-22 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 3-Phosphoglyceric acid GC-MS (2 TMS) - 70eV, Positive | splash10-00fu-9081000000-487a9a33cdad68046ff3 | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 3-Phosphoglyceric acid GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 3-Phosphoglyceric acid GC-MS (TMS_1_1) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 3-Phosphoglyceric acid GC-MS (TMS_1_2) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 3-Phosphoglyceric acid GC-MS (TMS_1_3) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 3-Phosphoglyceric acid GC-MS (TMS_2_2) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 3-Phosphoglyceric acid GC-MS (TMS_2_3) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 3-Phosphoglyceric acid GC-MS (TMS_2_4) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 3-Phosphoglyceric acid GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 3-Phosphoglyceric acid GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 3-Phosphoglyceric acid GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 3-Phosphoglyceric acid GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 3-Phosphoglyceric acid GC-MS (TBDMS_2_2) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 3-Phosphoglyceric acid GC-MS (TBDMS_2_3) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 3-Phosphoglyceric acid GC-MS (TBDMS_2_4) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental LC-MS/MS | LC-MS/MS Spectrum - 3-Phosphoglyceric acid Quattro_QQQ 10V, Negative-QTOF (Annotated) | splash10-002k-9300000000-4fafb3c93719c5a41327 | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 3-Phosphoglyceric acid Quattro_QQQ 25V, Negative-QTOF (Annotated) | splash10-004j-9000000000-c6bb9644e70a338c03be | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 3-Phosphoglyceric acid Quattro_QQQ 40V, Negative-QTOF (Annotated) | splash10-004i-9000000000-9d164579c98406ad7c88 | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 3-Phosphoglyceric acid LC-ESI-QQ (API3000, Applied Biosystems) 10V, Positive-QTOF | splash10-0a4i-0900000000-cea59554251398a27835 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 3-Phosphoglyceric acid LC-ESI-QQ (API3000, Applied Biosystems) 20V, Positive-QTOF | splash10-0udi-0900000000-8a976aaa2f56f074d975 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 3-Phosphoglyceric acid LC-ESI-QQ (API3000, Applied Biosystems) 30V, Positive-QTOF | splash10-0a5c-9500000000-4bd264fcf029200c55c6 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 3-Phosphoglyceric acid LC-ESI-QQ (API3000, Applied Biosystems) 40V, Positive-QTOF | splash10-001i-9100000000-1a020681f04042ff6966 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 3-Phosphoglyceric acid LC-ESI-QQ , positive-QTOF | splash10-0a4i-0900000000-cea59554251398a27835 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 3-Phosphoglyceric acid LC-ESI-QQ , positive-QTOF | splash10-0udi-0900000000-8a976aaa2f56f074d975 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 3-Phosphoglyceric acid LC-ESI-QQ , positive-QTOF | splash10-0a5c-9600000000-4bd264fcf029200c55c6 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 3-Phosphoglyceric acid LC-ESI-QQ , positive-QTOF | splash10-001i-9100000000-1a020681f04042ff6966 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 3-Phosphoglyceric acid , positive-QTOF | splash10-0005-8900000000-bd4e0c8adef56968d87a | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 3-Phosphoglyceric acid 30V, Negative-QTOF | splash10-004i-9100000000-abd08e7a88ff6e65bc4b | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 3-Phosphoglyceric acid 10V, Negative-QTOF | splash10-002b-9000000000-f8afa9e495e0f6c94717 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 3-Phosphoglyceric acid 30V, Negative-QTOF | splash10-004i-9200000000-30ac9f237fd5b07a8ad8 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 3-Phosphoglyceric acid 20V, Negative-QTOF | splash10-004j-9000000000-623d31045a6d2723a66b | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 3-Phosphoglyceric acid 35V, Positive-QTOF | splash10-0005-9800000000-592cc43b2d6718b5e805 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 3-Phosphoglyceric acid 40V, Negative-QTOF | splash10-004i-9000000000-75f21a1b3e228e0af7f2 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3-Phosphoglyceric acid 10V, Positive-QTOF | splash10-00kr-3900000000-9d9dc94243143123d53b | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3-Phosphoglyceric acid 20V, Positive-QTOF | splash10-00kv-8900000000-b21c05dfded763ce62a0 | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3-Phosphoglyceric acid 40V, Positive-QTOF | splash10-0006-9000000000-c460ca316484eef90129 | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3-Phosphoglyceric acid 10V, Negative-QTOF | splash10-003r-5900000000-653e5055531f19df4952 | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3-Phosphoglyceric acid 20V, Negative-QTOF | splash10-004i-9100000000-15195031d70847a75881 | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3-Phosphoglyceric acid 40V, Negative-QTOF | splash10-004i-9000000000-59550676899d259aeab5 | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3-Phosphoglyceric acid 10V, Negative-QTOF | splash10-004j-9000000000-0b41dc890757740bbb51 | 2021-09-22 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Experimental 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, experimental) | 2021-10-10 | Wishart Lab | View Spectrum | | Experimental 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, experimental) | 2021-10-10 | Wishart Lab | View Spectrum | | Experimental 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, experimental) | 2021-10-10 | Wishart Lab | View Spectrum | | Experimental 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | 2012-12-05 | Wishart Lab | View Spectrum |
IR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M-H]-) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+H]+) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+Na]+) | 2023-02-03 | FELIX lab | View Spectrum |
|
|---|
| General References | - Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM: Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009 Feb 12;457(7231):910-4. doi: 10.1038/nature07762. [PubMed:19212411 ]
- Nakayama Y, Kinoshita A, Tomita M: Dynamic simulation of red blood cell metabolism and its application to the analysis of a pathological condition. Theor Biol Med Model. 2005 May 9;2:18. [PubMed:15882454 ]
- Yu KT, Pendley C 2nd, Herczeg T, Pendleton RG: 2,3-Diphosphoglycerate phosphatase/synthase: a potential target for elevating the diphosphoglycerate level in human red blood cells. J Pharmacol Exp Ther. 1990 Jan;252(1):192-200. [PubMed:2153800 ]
- Uchida K, Kondoh K, Matuo Y: Recombinant M-, B- and MB-type isozymes of human phosphoglyceric acid mutase: their large-scale production and preparation of polyclonal antibodies specific to M- and B-type isozymes. Clin Chim Acta. 1995 Jun 15;237(1-2):43-58. [PubMed:7664478 ]
- Uchida K, Mori K, Matuo Y: [Phosphoglyceric acid mutase]. Nihon Rinsho. 1995 May;53(5):1247-52. [PubMed:7602787 ]
- Nakai A, Shigematsu Y, Liu YY, Kikawa Y, Sudo M: Urinary sugar phosphates and related organic acids in fructose-1,6-diphosphatase deficiency. J Inherit Metab Dis. 1993;16(2):408-14. [PubMed:8412001 ]
- Sayed A, Matsuyama S, Inoue K, Alsina J, Cai F, Chen J, Inouye M: ATPase and GTPase activities copurifying with GTP-binding proteins in E. coli. J Mol Microbiol Biotechnol. 2000 Jul;2(3):261-3. [PubMed:10937433 ]
- Grisolia S, Salinas M, Wallace R, Singh GK: Influence of size, protein concentration, protein synthesis inhibitors,and carbon on clearance of enzymes and proteins from blood. Physiol Chem Phys. 1976;8(1):37-52. [PubMed:183226 ]
- Mair J: Progress in myocardial damage detection: new biochemical markers for clinicians. Crit Rev Clin Lab Sci. 1997;34(1):1-66. [PubMed:9055056 ]
- Glaus M, Schneider W: Iron release from transferrin induced by mixed ligand complexes of copper(II). Biol Met. 1989;2(3):185-90. [PubMed:2490073 ]
- Veech RL, Lawson JW, Cornell NW, Krebs HA: Cytosolic phosphorylation potential. J Biol Chem. 1979 Jul 25;254(14):6538-47. [PubMed:36399 ]
- Elshenawy S, Pinney SE, Stuart T, Doulias PT, Zura G, Parry S, Elovitz MA, Bennett MJ, Bansal A, Strauss JF 3rd, Ischiropoulos H, Simmons RA: The Metabolomic Signature of the Placenta in Spontaneous Preterm Birth. Int J Mol Sci. 2020 Feb 4;21(3). pii: ijms21031043. doi: 10.3390/ijms21031043. [PubMed:32033212 ]
- Sun Q, Li S, Wang Y, Peng H, Zhang X, Zheng Y, Li C, Li L, Chen R, Chen X, Bai W, Jiang X, Liu L, Wei F, Wang B, Zhang Y, Li H, Ren X, Zhang H: Phosphoglyceric acid mutase-1 contributes to oncogenic mTOR-mediated tumor growth and confers non-small cell lung cancer patients with poor prognosis. Cell Death Differ. 2018 Jun;25(6):1160-1173. doi: 10.1038/s41418-017-0034-y. Epub 2018 Jan 23. [PubMed:29362480 ]
- (). Mayo Medical Laboratories 2005 Test Catalog. .
|
|---|