| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected and Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2023-02-21 17:15:20 UTC |
|---|
| HMDB ID | HMDB0000962 |
|---|
| Secondary Accession Numbers | - HMDB0006877
- HMDB0006950
- HMDB00962
- HMDB06877
- HMDB06950
|
|---|
| Metabolite Identification |
|---|
| Common Name | Lipoamide |
|---|
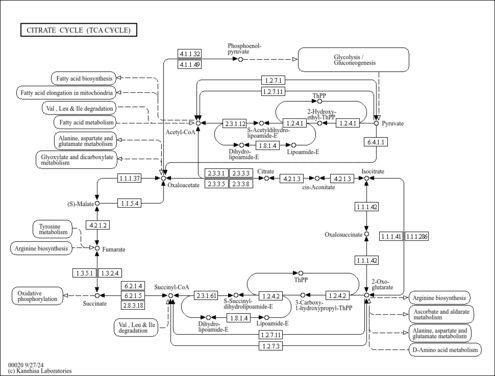
| Description | Lipoamide is a trivial name for 6,8-dithiooctanoic amide. It is 6,8-dithiooctanoic acid's functional form where the carboxyl group is attached to protein (or any other amine) by an amide linkage (containing -NH2) to an amino group. Lipoamide forms a thioester bond, oxidizing the disulfide bond, with acetaldehyde (pyruvate after it has been decarboxylated). It then transfers the acetaldehyde group to CoA which can then continue in the TCA cycle. Lipoamide is an intermediate in glycolysis/gluconeogenesis, citrate cycle (TCA cycle), alanine, aspartate and pyruvate metabolism, and valine, leucine and isoleucine degradation (KEGG:C00248). It is generated from dihydrolipoamide via the enzyme dihydrolipoamide dehydrogenase (EC:1.8.1.4) and then converted to S-glutaryl-dihydrolipoamide via the enzyme oxoglutarate dehydrogenase (EC:1.2.4.2). |
|---|
| Structure | InChI=1S/C8H15NOS2/c9-8(10)4-2-1-3-7-5-6-11-12-7/h7H,1-6H2,(H2,9,10) |
|---|
| Synonyms | | Value | Source |
|---|
| 1,2-Dithiolane-3-pentanamide | ChEBI | | alpha-Lipoic acid amide | ChEBI | | Lipoacin | ChEBI | | Thioctamide | ChEBI | | Thioctic acid amide | ChEBI | | Vitamin N | ChEBI | | a-Lipoate amide | Generator | | a-Lipoic acid amide | Generator | | alpha-Lipoate amide | Generator | | Α-lipoate amide | Generator | | Α-lipoic acid amide | Generator | | Thioctate amide | Generator | | 5-(1,2-Dithiolan-3-yl)-pentanamide | HMDB | | 5-(1,2-Dithiolan-3-yl)pentanamide | HMDB | | 5-(1,2-Dithiolan-3-yl)valeramide | HMDB | | 5-(Dithiolan-3-yl)valeramide | HMDB | | alpha-Lipoate | HMDB | | alpha-Lipoic acid | HMDB | | DL-6-Thioctic amide | HMDB | | DL-Lipoamide | HMDB | | Lipamide | HMDB | | Lipoamid | HMDB | | Lipoicin | HMDB | | Lipozyme | HMDB | | Lypoaran | HMDB | | Pathoclon | HMDB | | Thioami | HMDB | | Thioctamid | HMDB | | Thiotomin | HMDB | | Ticolin | HMDB | | 1,2-Dithiolane-3-valeramide | HMDB | | 6,8-Thioctic amide | HMDB | | alpha-Lipoic amide | HMDB | | Lipoamide, (+-)-isomer | HMDB |
|
|---|
| Chemical Formula | C8H15NOS2 |
|---|
| Average Molecular Weight | 205.341 |
|---|
| Monoisotopic Molecular Weight | 205.059505487 |
|---|
| IUPAC Name | 5-(1,2-dithiolan-3-yl)pentanamide |
|---|
| Traditional Name | lipoamide |
|---|
| CAS Registry Number | 940-69-2 |
|---|
| SMILES | NC(=O)CCCCC1CCSS1 |
|---|
| InChI Identifier | InChI=1S/C8H15NOS2/c9-8(10)4-2-1-3-7-5-6-11-12-7/h7H,1-6H2,(H2,9,10) |
|---|
| InChI Key | FCCDDURTIIUXBY-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as lipoamides. Lipoamides are compounds containing a lipoamide moiety, which consists of a pentanamide attached to the C3 carbon atom of a 1,2-dithiolane ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Dithiolanes |
|---|
| Sub Class | Lipoamides |
|---|
| Direct Parent | Lipoamides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Lipoamide
- Fatty amide
- Fatty acyl
- 1,2-dithiolane
- Carboxamide group
- Organic disulfide
- Primary carboxylic acid amide
- Carboxylic acid derivative
- Carbonyl group
- Organooxygen compound
- Organonitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | 126.0 - 129.0 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Experimental Collision Cross Sections |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Retention Times Underivatized| Chromatographic Method | Retention Time | Reference |
|---|
| Measured using a Waters Acquity ultraperformance liquid chromatography (UPLC) ethylene-bridged hybrid (BEH) C18 column (100 mm × 2.1 mm; 1.7 μmparticle diameter). Predicted by Afia on May 17, 2022. Predicted by Afia on May 17, 2022. | 3.43 minutes | 32390414 | | Predicted by Siyang on May 30, 2022 | 10.759 minutes | 33406817 | | Predicted by Siyang using ReTip algorithm on June 8, 2022 | 1.38 minutes | 32390414 | | AjsUoB = Accucore 150 Amide HILIC with 10mM Ammonium Formate, 0.1% Formic Acid | 35.5 seconds | 40023050 | | Fem_Long = Waters ACQUITY UPLC HSS T3 C18 with Water:MeOH and 0.1% Formic Acid | 1620.0 seconds | 40023050 | | Fem_Lipids = Ascentis Express C18 with (60:40 water:ACN):(90:10 IPA:ACN) and 10mM NH4COOH + 0.1% Formic Acid | 314.3 seconds | 40023050 | | Life_Old = Waters ACQUITY UPLC BEH C18 with Water:(20:80 acetone:ACN) and 0.1% Formic Acid | 129.2 seconds | 40023050 | | Life_New = RP Waters ACQUITY UPLC HSS T3 C18 with Water:(30:70 MeOH:ACN) and 0.1% Formic Acid | 188.8 seconds | 40023050 | | RIKEN = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 105.8 seconds | 40023050 | | Eawag_XBridgeC18 = XBridge C18 3.5u 2.1x50 mm with Water:MeOH and 0.1% Formic Acid | 332.4 seconds | 40023050 | | BfG_NTS_RP1 =Agilent Zorbax Eclipse Plus C18 (2.1 mm x 150 mm, 3.5 um) with Water:ACN and 0.1% Formic Acid | 379.3 seconds | 40023050 | | HILIC_BDD_2 = Merck SeQuant ZIC-HILIC with ACN(0.1% formic acid):water(16 mM ammonium formate) | 212.4 seconds | 40023050 | | UniToyama_Atlantis = RP Waters Atlantis T3 (2.1 x 150 mm, 5 um) with ACN:Water and 0.1% Formic Acid | 899.2 seconds | 40023050 | | BDD_C18 = Hypersil Gold 1.9µm C18 with Water:ACN and 0.1% Formic Acid | 361.3 seconds | 40023050 | | UFZ_Phenomenex = Kinetex Core-Shell C18 2.6 um, 3.0 x 100 mm, Phenomenex with Water:MeOH and 0.1% Formic Acid | 957.8 seconds | 40023050 | | SNU_RIKEN_POS = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 256.0 seconds | 40023050 | | RPMMFDA = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 261.7 seconds | 40023050 | | MTBLS87 = Merck SeQuant ZIC-pHILIC column with ACN:Water and :ammonium carbonate | 489.4 seconds | 40023050 | | KI_GIAR_zic_HILIC_pH2_7 = Merck SeQuant ZIC-HILIC with ACN:Water and 0.1% FA | 358.5 seconds | 40023050 | | Meister zic-pHILIC pH9.3 = Merck SeQuant ZIC-pHILIC column with ACN:Water 5mM NH4Ac pH9.3 and 5mM ammonium acetate in water | 146.3 seconds | 40023050 |
Predicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| Lipoamide,1TMS,isomer #1 | C[Si](C)(C)NC(=O)CCCCC1CCSS1 | 2087.7 | Semi standard non polar | 33892256 | | Lipoamide,1TMS,isomer #1 | C[Si](C)(C)NC(=O)CCCCC1CCSS1 | 1987.5 | Standard non polar | 33892256 | | Lipoamide,1TMS,isomer #1 | C[Si](C)(C)NC(=O)CCCCC1CCSS1 | 3194.8 | Standard polar | 33892256 | | Lipoamide,2TMS,isomer #1 | C[Si](C)(C)N(C(=O)CCCCC1CCSS1)[Si](C)(C)C | 2286.1 | Semi standard non polar | 33892256 | | Lipoamide,2TMS,isomer #1 | C[Si](C)(C)N(C(=O)CCCCC1CCSS1)[Si](C)(C)C | 2155.5 | Standard non polar | 33892256 | | Lipoamide,2TMS,isomer #1 | C[Si](C)(C)N(C(=O)CCCCC1CCSS1)[Si](C)(C)C | 3001.9 | Standard polar | 33892256 | | Lipoamide,1TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)NC(=O)CCCCC1CCSS1 | 2315.7 | Semi standard non polar | 33892256 | | Lipoamide,1TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)NC(=O)CCCCC1CCSS1 | 2215.5 | Standard non polar | 33892256 | | Lipoamide,1TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)NC(=O)CCCCC1CCSS1 | 3128.6 | Standard polar | 33892256 | | Lipoamide,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)N(C(=O)CCCCC1CCSS1)[Si](C)(C)C(C)(C)C | 2663.0 | Semi standard non polar | 33892256 | | Lipoamide,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)N(C(=O)CCCCC1CCSS1)[Si](C)(C)C(C)(C)C | 2617.0 | Standard non polar | 33892256 | | Lipoamide,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)N(C(=O)CCCCC1CCSS1)[Si](C)(C)C(C)(C)C | 2886.2 | Standard polar | 33892256 |
|
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental GC-MS | GC-MS Spectrum - Lipoamide GC-MS (2 TMS) | splash10-014i-6963000000-7e1887d53c2801b09961 | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Lipoamide GC-MS (1 TMS) | splash10-017l-3920000000-a3a7a08acf06d41c9b73 | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Lipoamide GC-MS (Non-derivatized) | splash10-014i-6963000000-7e1887d53c2801b09961 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Lipoamide GC-MS (Non-derivatized) | splash10-017l-3920000000-a3a7a08acf06d41c9b73 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Lipoamide GC-EI-TOF (Non-derivatized) | splash10-014i-1910000000-b6ee336856e354af8114 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Lipoamide GC-EI-TOF (Non-derivatized) | splash10-00di-3900000000-60cad9dd198c3ad64ed7 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Lipoamide GC-MS (Non-derivatized) - 70eV, Positive | splash10-0f96-9800000000-ac967ad86beefc26c983 | 2016-09-22 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Lipoamide GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental LC-MS/MS | LC-MS/MS Spectrum - Lipoamide Quattro_QQQ 10V, Positive-QTOF (Annotated) | splash10-000i-0900000000-24fca44fc025dbd6be18 | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Lipoamide Quattro_QQQ 25V, Positive-QTOF (Annotated) | splash10-0ziu-9700000000-7dfd23f450bdfd581100 | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Lipoamide Quattro_QQQ 40V, Positive-QTOF (Annotated) | splash10-056u-9000000000-13583c4eb6b06a56c392 | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Lipoamide LC-ESI-QQ (API3000, Applied Biosystems) 10V, Positive-QTOF | splash10-052r-2920000000-a4fb7176abf63e581f5e | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Lipoamide LC-ESI-QQ (API3000, Applied Biosystems) 20V, Positive-QTOF | splash10-000i-2900000000-10a079a84f024494ae23 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Lipoamide LC-ESI-QQ (API3000, Applied Biosystems) 30V, Positive-QTOF | splash10-0zfr-9800000000-b01332176d6829b1eaa1 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Lipoamide LC-ESI-QQ (API3000, Applied Biosystems) 40V, Positive-QTOF | splash10-0udl-9400000000-7a03c741edae36dff9cf | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Lipoamide LC-ESI-QQ (API3000, Applied Biosystems) 50V, Positive-QTOF | splash10-0a5c-9100000000-7b44dfbfcbb0cc516254 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Lipoamide LC-ESI-IT (LC/MSD Trap XCT, Agilent Technologies) , Positive-QTOF | splash10-000i-0900000000-50512172ce038bce2995 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Lipoamide LC-ESI-IT (LC/MSD Trap XCT, Agilent Technologies) , Positive-QTOF | splash10-03di-0900000000-7652a33c17a37b6914aa | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Lipoamide LC-ESI-IT (LC/MSD Trap XCT, Agilent Technologies) , Positive-QTOF | splash10-0udi-0900000000-0df59a6540bede8a4a79 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Lipoamide LC-ESI-QQ , positive-QTOF | splash10-052r-2920000000-a4fb7176abf63e581f5e | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Lipoamide LC-ESI-QQ , positive-QTOF | splash10-000i-2900000000-158c946037c5d7bb3e50 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Lipoamide LC-ESI-QQ , positive-QTOF | splash10-0zfr-9800000000-b01332176d6829b1eaa1 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Lipoamide LC-ESI-QQ , positive-QTOF | splash10-0udl-9400000000-7a03c741edae36dff9cf | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Lipoamide LC-ESI-QQ , positive-QTOF | splash10-0a5c-9100000000-7b44dfbfcbb0cc516254 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Lipoamide LC-ESI-IT , positive-QTOF | splash10-000i-0900000000-fa779b1c88469a732321 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Lipoamide , positive-QTOF | splash10-000i-1900000000-9e4b6873b24c66cf9f69 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Lipoamide 10V, Positive-QTOF | splash10-01p9-0900000000-b37ce6b25b9feef09b96 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Lipoamide 10V, Positive-QTOF | splash10-0a4r-0940000000-d79e1aa52057e567d71f | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Lipoamide 20V, Positive-QTOF | splash10-052r-3900000000-abd8148048c5f282d889 | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Lipoamide 40V, Positive-QTOF | splash10-0adr-9500000000-9769c619660ab6af46d2 | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Lipoamide 10V, Negative-QTOF | splash10-0zml-1920000000-79d57d488cb8fbc804a3 | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Lipoamide 20V, Negative-QTOF | splash10-0fdo-4910000000-d264eaf6e242c36bc563 | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Lipoamide 40V, Negative-QTOF | splash10-0006-9000000000-289d2f20046f7819a7ca | 2016-09-12 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Experimental 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, CD3OD, experimental) | 2012-12-05 | Wishart Lab | View Spectrum |
IR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M-H]-) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+H]+) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+Na]+) | 2023-02-03 | FELIX lab | View Spectrum |
|
|---|
| General References | - Arner ES, Nordberg J, Holmgren A: Efficient reduction of lipoamide and lipoic acid by mammalian thioredoxin reductase. Biochem Biophys Res Commun. 1996 Aug 5;225(1):268-74. [PubMed:8769129 ]
- Nordman T, Xia L, Bjorkhem-Bergman L, Damdimopoulos A, Nalvarte I, Arner ES, Spyrou G, Eriksson LC, Bjornstedt M, Olsson JM: Regeneration of the antioxidant ubiquinol by lipoamide dehydrogenase, thioredoxin reductase and glutathione reductase. Biofactors. 2003;18(1-4):45-50. [PubMed:14695919 ]
- Oizumi J, Hayakawa K: Liberation of lipoate by human serum lipoamidase from bovine heart pyruvate dehydrogenase. Biochem Biophys Res Commun. 1989 Jul 31;162(2):658-63. [PubMed:2502979 ]
- Nakai T, Nakagawa N, Maoka N, Masui R, Kuramitsu S, Kamiya N: Ligand-induced conformational changes and a reaction intermediate in branched-chain 2-oxo acid dehydrogenase (E1) from Thermus thermophilus HB8, as revealed by X-ray crystallography. J Mol Biol. 2004 Apr 2;337(4):1011-33. [PubMed:15033367 ]
- Wynn RM, Machius M, Chuang JL, Li J, Tomchick DR, Chuang DT: Roles of His291-alpha and His146-beta' in the reductive acylation reaction catalyzed by human branched-chain alpha-ketoacid dehydrogenase: refined phosphorylation loop structure in the active site. J Biol Chem. 2003 Oct 31;278(44):43402-10. Epub 2003 Aug 5. [PubMed:12902323 ]
- Panak KC, Ruiz OA, Giorgieri SA, Diaz LE: Direct determination of glutathione in human blood by micellar electrokinetic chromatography: simultaneous determination of lipoamide and lipoic acid. Electrophoresis. 1996 Oct;17(10):1613-6. [PubMed:8957191 ]
|
|---|