| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Expected but not Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2022-03-07 02:49:10 UTC |
|---|
| HMDB ID | HMDB0001457 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 5beta-Cholestane-3alpha,7alpha,12alpha-triol |
|---|
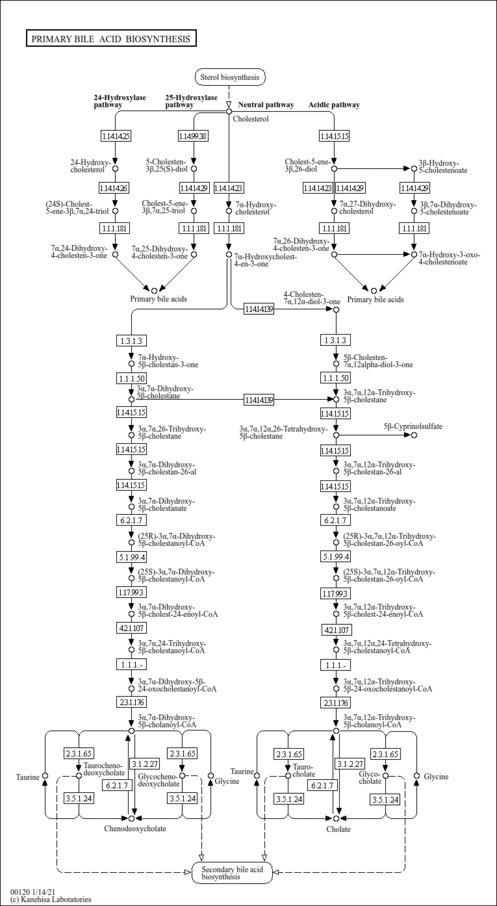
| Description | 5beta-Cholestane-3alpha,7alpha,12alpha-triol is an intermediate in bile acid biosynthesis. 5beta-Cholestane-3alpha,7alpha,12alpha-triol is the second to last step in the synthesis of 5beta-cyprinolsulfate. It is converted from 7alpha,12alpha-dihydroxy-5beta-cholestan-3-one via enzymatic reaction, and then it is converted into 3alpha,7alpha,12alpha,26-tetrahydroxy-5beta-cholestane via the enzyme cytochrome P450 (EC 1.14.13.15). This compound inhibits la-hydroxylation (PMID: 7937829 ). It is the byproduct of cholestanetetraol 26-dehydrogenase (EC 1.1.1.161) and the reaction that catalyzes it is classified as a small molecule reaction (BioCyc). |
|---|
| Structure | [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])C[C@H](O)[C@]12C)[C@H](C)CCCC(C)C InChI=1S/C27H48O3/c1-16(2)7-6-8-17(3)20-9-10-21-25-22(15-24(30)27(20,21)5)26(4)12-11-19(28)13-18(26)14-23(25)29/h16-25,28-30H,6-15H2,1-5H3/t17-,18+,19-,20-,21+,22+,23-,24+,25+,26+,27-/m1/s1 |
|---|
| Synonyms | | Value | Source |
|---|
| 3alpha,7alpha,12alpha-Trihydroxy-5beta-cholestane | ChEBI | | 3alpha,7alpha,12alpha-Trihydroxycoprostane | ChEBI | | 3a,7a,12a-Trihydroxy-5b-cholestane | Generator | | 3Α,7α,12α-trihydroxy-5β-cholestane | Generator | | 3a,7a,12a-Trihydroxycoprostane | Generator | | 3Α,7α,12α-trihydroxycoprostane | Generator | | 5b-Cholestane-3a,7a,12a-triol | Generator | | 5Β-cholestane-3α,7α,12α-triol | Generator | | 5-beta-Cholestane-3-alpha,7-alpha,12-alpha-triol | HMDB | | 3,7,12-Trihydroxycholestane | MeSH, HMDB | | 3,7,12-Trihydroxycoprostane | MeSH, HMDB | | 3,7,12-Trihydroxycoprostane, (3alpha,5alpha,7alpha,12alpha)-isomer | MeSH, HMDB | | 3,7,12-Trihydroxycoprostane, (3beta,5alpha,7alpha,12alpha)-isomer | MeSH, HMDB | | 3,7,12-Trihydroxycoprostane, (3beta,5beta,7alpha,12alpha)-isomer | MeSH, HMDB | | 3alpha, 7alpha,12alpha-Trihydroxy-5beta-cholestane | MeSH, HMDB | | 5 beta-Cholestane-3 alpha,7 alpha,12 alpha-triol | MeSH, HMDB | | 5beta-Cholestane-3alpha,7alpha,12alpha-triol | MeSH, KEGG | | Chtriol | MeSH, HMDB | | Trihydroxycoprostane | MeSH, HMDB | | (3alpha,5beta,7alpha,12alpha)-Cholestane-3,7,12-triol | HMDB | | (3α,5β,7α,12α)-Cholestane-3,7,12-triol | HMDB | | 5beta-Cholestan-3alpha,7alpha,12alpha-triol | HMDB | | 5β-Cholestan-3α,7α,12α-triol | HMDB |
|
|---|
| Chemical Formula | C27H48O3 |
|---|
| Average Molecular Weight | 420.6682 |
|---|
| Monoisotopic Molecular Weight | 420.360345402 |
|---|
| IUPAC Name | (1S,2S,5R,7S,9R,10R,11S,14R,15R,16S)-2,15-dimethyl-14-[(2R)-6-methylheptan-2-yl]tetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadecane-5,9,16-triol |
|---|
| Traditional Name | (1S,2S,5R,7S,9R,10R,11S,14R,15R,16S)-2,15-dimethyl-14-[(2R)-6-methylheptan-2-yl]tetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadecane-5,9,16-triol |
|---|
| CAS Registry Number | 547-96-6 |
|---|
| SMILES | [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])C[C@H](O)[C@]12C)[C@H](C)CCCC(C)C |
|---|
| InChI Identifier | InChI=1S/C27H48O3/c1-16(2)7-6-8-17(3)20-9-10-21-25-22(15-24(30)27(20,21)5)26(4)12-11-19(28)13-18(26)14-23(25)29/h16-25,28-30H,6-15H2,1-5H3/t17-,18+,19-,20-,21+,22+,23-,24+,25+,26+,27-/m1/s1 |
|---|
| InChI Key | RIVQQZVHIVNQFH-XJZYBRFWSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as cholesterols and derivatives. Cholesterols and derivatives are compounds containing a 3-hydroxylated cholestane core. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Cholestane steroids |
|---|
| Direct Parent | Cholesterols and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cholesterol-skeleton
- Cholesterol
- 7-hydroxysteroid
- 3-alpha-hydroxysteroid
- Hydroxysteroid
- 12-hydroxysteroid
- 3-hydroxysteroid
- Cyclic alcohol
- Secondary alcohol
- Polyol
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Not Available |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| 5beta-Cholestane-3alpha,7alpha,12alpha-triol,1TMS,isomer #1 | CC(C)CCC[C@@H](C)[C@H]1CC[C@H]2[C@H]3[C@H](C[C@H](O)[C@@]21C)[C@@]1(C)CC[C@@H](O)C[C@H]1C[C@H]3O[Si](C)(C)C | 3231.6 | Semi standard non polar | 33892256 | | 5beta-Cholestane-3alpha,7alpha,12alpha-triol,1TMS,isomer #2 | CC(C)CCC[C@@H](C)[C@H]1CC[C@H]2[C@H]3[C@H](C[C@H](O)[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C)C[C@H]1C[C@H]3O | 3291.0 | Semi standard non polar | 33892256 | | 5beta-Cholestane-3alpha,7alpha,12alpha-triol,1TMS,isomer #3 | CC(C)CCC[C@@H](C)[C@H]1CC[C@H]2[C@H]3[C@H](C[C@H](O[Si](C)(C)C)[C@@]21C)[C@@]1(C)CC[C@@H](O)C[C@H]1C[C@H]3O | 3292.1 | Semi standard non polar | 33892256 | | 5beta-Cholestane-3alpha,7alpha,12alpha-triol,2TMS,isomer #1 | CC(C)CCC[C@@H](C)[C@H]1CC[C@H]2[C@H]3[C@H](C[C@H](O[Si](C)(C)C)[C@@]21C)[C@@]1(C)CC[C@@H](O)C[C@H]1C[C@H]3O[Si](C)(C)C | 3242.8 | Semi standard non polar | 33892256 | | 5beta-Cholestane-3alpha,7alpha,12alpha-triol,2TMS,isomer #2 | CC(C)CCC[C@@H](C)[C@H]1CC[C@H]2[C@H]3[C@H](C[C@H](O)[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C)C[C@H]1C[C@H]3O[Si](C)(C)C | 3222.0 | Semi standard non polar | 33892256 | | 5beta-Cholestane-3alpha,7alpha,12alpha-triol,2TMS,isomer #3 | CC(C)CCC[C@@H](C)[C@H]1CC[C@H]2[C@H]3[C@H](C[C@H](O[Si](C)(C)C)[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C)C[C@H]1C[C@H]3O | 3242.3 | Semi standard non polar | 33892256 | | 5beta-Cholestane-3alpha,7alpha,12alpha-triol,3TMS,isomer #1 | CC(C)CCC[C@@H](C)[C@H]1CC[C@H]2[C@H]3[C@H](C[C@H](O[Si](C)(C)C)[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C)C[C@H]1C[C@H]3O[Si](C)(C)C | 3252.2 | Semi standard non polar | 33892256 | | 5beta-Cholestane-3alpha,7alpha,12alpha-triol,1TBDMS,isomer #1 | CC(C)CCC[C@@H](C)[C@H]1CC[C@H]2[C@H]3[C@H](C[C@H](O)[C@@]21C)[C@@]1(C)CC[C@@H](O)C[C@H]1C[C@H]3O[Si](C)(C)C(C)(C)C | 3465.3 | Semi standard non polar | 33892256 | | 5beta-Cholestane-3alpha,7alpha,12alpha-triol,1TBDMS,isomer #2 | CC(C)CCC[C@@H](C)[C@H]1CC[C@H]2[C@H]3[C@H](C[C@H](O)[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C(C)(C)C)C[C@H]1C[C@H]3O | 3528.7 | Semi standard non polar | 33892256 | | 5beta-Cholestane-3alpha,7alpha,12alpha-triol,1TBDMS,isomer #3 | CC(C)CCC[C@@H](C)[C@H]1CC[C@H]2[C@H]3[C@H](C[C@H](O[Si](C)(C)C(C)(C)C)[C@@]21C)[C@@]1(C)CC[C@@H](O)C[C@H]1C[C@H]3O | 3531.8 | Semi standard non polar | 33892256 | | 5beta-Cholestane-3alpha,7alpha,12alpha-triol,2TBDMS,isomer #1 | CC(C)CCC[C@@H](C)[C@H]1CC[C@H]2[C@H]3[C@H](C[C@H](O[Si](C)(C)C(C)(C)C)[C@@]21C)[C@@]1(C)CC[C@@H](O)C[C@H]1C[C@H]3O[Si](C)(C)C(C)(C)C | 3701.5 | Semi standard non polar | 33892256 | | 5beta-Cholestane-3alpha,7alpha,12alpha-triol,2TBDMS,isomer #2 | CC(C)CCC[C@@H](C)[C@H]1CC[C@H]2[C@H]3[C@H](C[C@H](O)[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C(C)(C)C)C[C@H]1C[C@H]3O[Si](C)(C)C(C)(C)C | 3668.3 | Semi standard non polar | 33892256 | | 5beta-Cholestane-3alpha,7alpha,12alpha-triol,2TBDMS,isomer #3 | CC(C)CCC[C@@H](C)[C@H]1CC[C@H]2[C@H]3[C@H](C[C@H](O[Si](C)(C)C(C)(C)C)[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C(C)(C)C)C[C@H]1C[C@H]3O | 3706.5 | Semi standard non polar | 33892256 | | 5beta-Cholestane-3alpha,7alpha,12alpha-triol,3TBDMS,isomer #1 | CC(C)CCC[C@@H](C)[C@H]1CC[C@H]2[C@H]3[C@H](C[C@H](O[Si](C)(C)C(C)(C)C)[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C(C)(C)C)C[C@H]1C[C@H]3O[Si](C)(C)C(C)(C)C | 3930.5 | Semi standard non polar | 33892256 |
|
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - 5beta-Cholestane-3alpha,7alpha,12alpha-triol GC-MS (Non-derivatized) - 70eV, Positive | splash10-0kkc-0349300000-7324287f0b234066bd64 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 5beta-Cholestane-3alpha,7alpha,12alpha-triol GC-MS (3 TMS) - 70eV, Positive | splash10-00di-3210079000-e1f273aa178918c83174 | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 5beta-Cholestane-3alpha,7alpha,12alpha-triol GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 5beta-Cholestane-3alpha,7alpha,12alpha-triol 10V, Positive-QTOF | splash10-0udr-0007900000-f9ed4dff04df44d2af49 | 2016-08-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 5beta-Cholestane-3alpha,7alpha,12alpha-triol 20V, Positive-QTOF | splash10-0f79-2209400000-84e3ed6130fdfe17578f | 2016-08-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 5beta-Cholestane-3alpha,7alpha,12alpha-triol 40V, Positive-QTOF | splash10-00di-5209000000-1166d5a4d66dea526dfc | 2016-08-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 5beta-Cholestane-3alpha,7alpha,12alpha-triol 10V, Negative-QTOF | splash10-014i-0001900000-766e9dee4a7aba343c98 | 2016-08-03 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 5beta-Cholestane-3alpha,7alpha,12alpha-triol 20V, Negative-QTOF | splash10-0uxr-0001900000-90680fd53f8266be1ace | 2016-08-03 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 5beta-Cholestane-3alpha,7alpha,12alpha-triol 40V, Negative-QTOF | splash10-0f79-2209400000-a1dd0a941d753b0d75e7 | 2016-08-03 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 5beta-Cholestane-3alpha,7alpha,12alpha-triol 10V, Negative-QTOF | splash10-014i-0000900000-722417cd9cab9945ab4a | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 5beta-Cholestane-3alpha,7alpha,12alpha-triol 20V, Negative-QTOF | splash10-014i-0000900000-722417cd9cab9945ab4a | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 5beta-Cholestane-3alpha,7alpha,12alpha-triol 40V, Negative-QTOF | splash10-014i-0010900000-aeb99a2e383b60fbc744 | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 5beta-Cholestane-3alpha,7alpha,12alpha-triol 10V, Positive-QTOF | splash10-00di-0001900000-f143819ead264249729e | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 5beta-Cholestane-3alpha,7alpha,12alpha-triol 20V, Positive-QTOF | splash10-0abi-9122200000-2f2c0b60a02fc711aa0c | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 5beta-Cholestane-3alpha,7alpha,12alpha-triol 40V, Positive-QTOF | splash10-0a4i-9721000000-6687d50233adfbaf7604 | 2021-09-24 | Wishart Lab | View Spectrum |
|
|---|