| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected and Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2023-02-21 17:15:13 UTC |
|---|
| HMDB ID | HMDB0000828 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Ureidosuccinic acid |
|---|
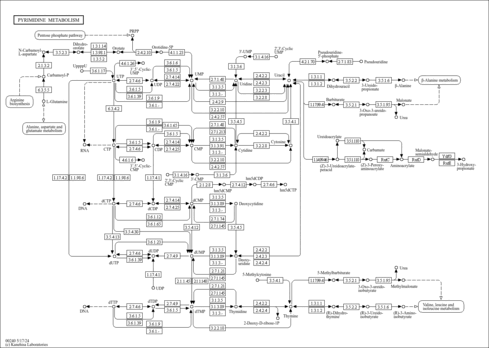
| Description | Ureidosuccinic acid, also known as L-ureidosuccinate or carbamyl-L-aspartate, belongs to the class of organic compounds known as aspartic acids and derivatives. Aspartic acids and derivatives are compounds containing an aspartic acid or a derivative thereof resulting from reaction of aspartic acid at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. Ureidosuccinic acid is also classified as a carbamate derivative. It is a solid that is soluble in water. Ureidosuccinic acid exists in all living species, ranging from bacteria to plants to humans. Ureidosuccinic acid can be biosynthesized from carbamoyl phosphate and L-aspartic acid through the action of the enzyme known as aspartate carbamoyltransferase (ACTase) and serves as an intermediate in pyrimidine biosynthesis. In humans, a drop in the level of urinary ureidosuccinic acid is associated with bladder cancer (PMID: 25562196 ). It is also involved in the metabolic disorder called Canavan disease. |
|---|
| Structure | NC(=O)N[C@@H](CC(O)=O)C(O)=O InChI=1S/C5H8N2O5/c6-5(12)7-2(4(10)11)1-3(8)9/h2H,1H2,(H,8,9)(H,10,11)(H3,6,7,12)/t2-/m0/s1 |
|---|
| Synonyms | | Value | Source |
|---|
| Carbamyl-L-aspartic acid | ChEBI | | L-Ureidosuccinic acid | ChEBI | | N-(Aminocarbonyl)-L-aspartic acid | ChEBI | | N-Carbamoyl-L-aspartate | ChEBI | | N-Carbamoyl-S-aspartic acid | ChEBI | | Carbamyl-L-aspartate | Generator | | L-Ureidosuccinate | Generator | | N-(Aminocarbonyl)-L-aspartate | Generator | | N-Carbamoyl-L-aspartic acid | Generator | | N-Carbamoyl-S-aspartate | Generator | | Ureidosuccinate | Generator | | 2-Ureidobutanedioate | HMDB | | 2-Ureidobutanedioic acid | HMDB | | Carbamoylaspartic acid | HMDB | | Carbamylaspartic acid | HMDB | | L-N-Carbamoylaspartic acid | HMDB | | N-Carbamoylaspartate | HMDB | | N-Carbamoylaspartic acid | HMDB | | NCD | HMDB | | Carbamyl-DL-aspartate | HMDB | | Ureidosuccinic acid, (D)-isomer | HMDB | | Ureidosuccinic acid, maganeese (+2), (1:1) salt | HMDB | | Ureidosuccinic acid, zinc (1:1) salt, (L)-isomer | HMDB | | N-Carbamoyl-D-aspartic acid | HMDB | | Ureidosuccinic acid, (L)-isomer | HMDB | | Ureidosuccinic acid, cobalt (+2), (1:1) salt,(L)-isomer | HMDB |
|
|---|
| Chemical Formula | C5H8N2O5 |
|---|
| Average Molecular Weight | 176.1274 |
|---|
| Monoisotopic Molecular Weight | 176.043321376 |
|---|
| IUPAC Name | (2S)-2-(carbamoylamino)butanedioic acid |
|---|
| Traditional Name | carbamylaspartic acid |
|---|
| CAS Registry Number | 13184-27-5 |
|---|
| SMILES | NC(=O)N[C@@H](CC(O)=O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C5H8N2O5/c6-5(12)7-2(4(10)11)1-3(8)9/h2H,1H2,(H,8,9)(H,10,11)(H3,6,7,12)/t2-/m0/s1 |
|---|
| InChI Key | HLKXYZVTANABHZ-REOHCLBHSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as aspartic acid and derivatives. Aspartic acid and derivatives are compounds containing an aspartic acid or a derivative thereof resulting from reaction of aspartic acid at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Aspartic acid and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Aspartic acid or derivatives
- Dicarboxylic acid or derivatives
- Fatty acid
- Isourea
- Carboximidamide
- Carboxylic acid
- Carboximidic acid derivative
- Organic oxide
- Organic nitrogen compound
- Hydrocarbon derivative
- Carbonyl group
- Organooxygen compound
- Organonitrogen compound
- Imine
- Organopnictogen compound
- Organic oxygen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | 174 - 175 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 3.7 mg/mL | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Not Available |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Retention Times Underivatized| Chromatographic Method | Retention Time | Reference |
|---|
| Measured using a Waters Acquity ultraperformance liquid chromatography (UPLC) ethylene-bridged hybrid (BEH) C18 column (100 mm × 2.1 mm; 1.7 μmparticle diameter). Predicted by Afia on May 17, 2022. Predicted by Afia on May 17, 2022. | 1.51 minutes | 32390414 | | Predicted by Siyang on May 30, 2022 | 10.0651 minutes | 33406817 | | Predicted by Siyang using ReTip algorithm on June 8, 2022 | 7.71 minutes | 32390414 | | AjsUoB = Accucore 150 Amide HILIC with 10mM Ammonium Formate, 0.1% Formic Acid | 443.5 seconds | 40023050 | | Fem_Long = Waters ACQUITY UPLC HSS T3 C18 with Water:MeOH and 0.1% Formic Acid | 467.6 seconds | 40023050 | | Fem_Lipids = Ascentis Express C18 with (60:40 water:ACN):(90:10 IPA:ACN) and 10mM NH4COOH + 0.1% Formic Acid | 354.0 seconds | 40023050 | | Life_Old = Waters ACQUITY UPLC BEH C18 with Water:(20:80 acetone:ACN) and 0.1% Formic Acid | 25.2 seconds | 40023050 | | Life_New = RP Waters ACQUITY UPLC HSS T3 C18 with Water:(30:70 MeOH:ACN) and 0.1% Formic Acid | 219.1 seconds | 40023050 | | RIKEN = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 101.5 seconds | 40023050 | | Eawag_XBridgeC18 = XBridge C18 3.5u 2.1x50 mm with Water:MeOH and 0.1% Formic Acid | 320.7 seconds | 40023050 | | BfG_NTS_RP1 =Agilent Zorbax Eclipse Plus C18 (2.1 mm x 150 mm, 3.5 um) with Water:ACN and 0.1% Formic Acid | 220.8 seconds | 40023050 | | HILIC_BDD_2 = Merck SeQuant ZIC-HILIC with ACN(0.1% formic acid):water(16 mM ammonium formate) | 936.4 seconds | 40023050 | | UniToyama_Atlantis = RP Waters Atlantis T3 (2.1 x 150 mm, 5 um) with ACN:Water and 0.1% Formic Acid | 615.1 seconds | 40023050 | | BDD_C18 = Hypersil Gold 1.9µm C18 with Water:ACN and 0.1% Formic Acid | 46.2 seconds | 40023050 | | UFZ_Phenomenex = Kinetex Core-Shell C18 2.6 um, 3.0 x 100 mm, Phenomenex with Water:MeOH and 0.1% Formic Acid | 745.7 seconds | 40023050 | | SNU_RIKEN_POS = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 222.7 seconds | 40023050 | | RPMMFDA = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 374.7 seconds | 40023050 | | MTBLS87 = Merck SeQuant ZIC-pHILIC column with ACN:Water and :ammonium carbonate | 748.9 seconds | 40023050 | | KI_GIAR_zic_HILIC_pH2_7 = Merck SeQuant ZIC-HILIC with ACN:Water and 0.1% FA | 520.3 seconds | 40023050 | | Meister zic-pHILIC pH9.3 = Merck SeQuant ZIC-pHILIC column with ACN:Water 5mM NH4Ac pH9.3 and 5mM ammonium acetate in water | 505.5 seconds | 40023050 |
Predicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| Ureidosuccinic acid,1TMS,isomer #1 | C[Si](C)(C)OC(=O)C[C@H](NC(N)=O)C(=O)O | 1771.3 | Semi standard non polar | 33892256 | | Ureidosuccinic acid,1TMS,isomer #2 | C[Si](C)(C)OC(=O)[C@H](CC(=O)O)NC(N)=O | 1718.2 | Semi standard non polar | 33892256 | | Ureidosuccinic acid,1TMS,isomer #3 | C[Si](C)(C)NC(=O)N[C@@H](CC(=O)O)C(=O)O | 1830.2 | Semi standard non polar | 33892256 | | Ureidosuccinic acid,1TMS,isomer #4 | C[Si](C)(C)N(C(N)=O)[C@@H](CC(=O)O)C(=O)O | 1807.8 | Semi standard non polar | 33892256 | | Ureidosuccinic acid,2TMS,isomer #1 | C[Si](C)(C)OC(=O)C[C@H](NC(N)=O)C(=O)O[Si](C)(C)C | 1848.9 | Semi standard non polar | 33892256 | | Ureidosuccinic acid,2TMS,isomer #2 | C[Si](C)(C)NC(=O)N[C@@H](CC(=O)O[Si](C)(C)C)C(=O)O | 1902.5 | Semi standard non polar | 33892256 | | Ureidosuccinic acid,2TMS,isomer #3 | C[Si](C)(C)OC(=O)C[C@@H](C(=O)O)N(C(N)=O)[Si](C)(C)C | 1796.3 | Semi standard non polar | 33892256 | | Ureidosuccinic acid,2TMS,isomer #4 | C[Si](C)(C)NC(=O)N[C@@H](CC(=O)O)C(=O)O[Si](C)(C)C | 1875.1 | Semi standard non polar | 33892256 | | Ureidosuccinic acid,2TMS,isomer #5 | C[Si](C)(C)OC(=O)[C@H](CC(=O)O)N(C(N)=O)[Si](C)(C)C | 1776.7 | Semi standard non polar | 33892256 | | Ureidosuccinic acid,2TMS,isomer #6 | C[Si](C)(C)N(C(=O)N[C@@H](CC(=O)O)C(=O)O)[Si](C)(C)C | 1941.3 | Semi standard non polar | 33892256 | | Ureidosuccinic acid,2TMS,isomer #7 | C[Si](C)(C)NC(=O)N([C@@H](CC(=O)O)C(=O)O)[Si](C)(C)C | 1878.7 | Semi standard non polar | 33892256 | | Ureidosuccinic acid,3TMS,isomer #1 | C[Si](C)(C)NC(=O)N[C@@H](CC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C | 1955.4 | Semi standard non polar | 33892256 | | Ureidosuccinic acid,3TMS,isomer #1 | C[Si](C)(C)NC(=O)N[C@@H](CC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C | 1747.6 | Standard non polar | 33892256 | | Ureidosuccinic acid,3TMS,isomer #1 | C[Si](C)(C)NC(=O)N[C@@H](CC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C | 2608.7 | Standard polar | 33892256 | | Ureidosuccinic acid,3TMS,isomer #2 | C[Si](C)(C)OC(=O)C[C@@H](C(=O)O[Si](C)(C)C)N(C(N)=O)[Si](C)(C)C | 1793.6 | Semi standard non polar | 33892256 | | Ureidosuccinic acid,3TMS,isomer #2 | C[Si](C)(C)OC(=O)C[C@@H](C(=O)O[Si](C)(C)C)N(C(N)=O)[Si](C)(C)C | 1764.7 | Standard non polar | 33892256 | | Ureidosuccinic acid,3TMS,isomer #2 | C[Si](C)(C)OC(=O)C[C@@H](C(=O)O[Si](C)(C)C)N(C(N)=O)[Si](C)(C)C | 2820.2 | Standard polar | 33892256 | | Ureidosuccinic acid,3TMS,isomer #3 | C[Si](C)(C)OC(=O)C[C@H](NC(=O)N([Si](C)(C)C)[Si](C)(C)C)C(=O)O | 1963.8 | Semi standard non polar | 33892256 | | Ureidosuccinic acid,3TMS,isomer #3 | C[Si](C)(C)OC(=O)C[C@H](NC(=O)N([Si](C)(C)C)[Si](C)(C)C)C(=O)O | 1872.7 | Standard non polar | 33892256 | | Ureidosuccinic acid,3TMS,isomer #3 | C[Si](C)(C)OC(=O)C[C@H](NC(=O)N([Si](C)(C)C)[Si](C)(C)C)C(=O)O | 2670.5 | Standard polar | 33892256 | | Ureidosuccinic acid,3TMS,isomer #4 | C[Si](C)(C)NC(=O)N([C@@H](CC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C | 1857.9 | Semi standard non polar | 33892256 | | Ureidosuccinic acid,3TMS,isomer #4 | C[Si](C)(C)NC(=O)N([C@@H](CC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C | 1866.7 | Standard non polar | 33892256 | | Ureidosuccinic acid,3TMS,isomer #4 | C[Si](C)(C)NC(=O)N([C@@H](CC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C | 2516.2 | Standard polar | 33892256 | | Ureidosuccinic acid,3TMS,isomer #5 | C[Si](C)(C)OC(=O)[C@H](CC(=O)O)NC(=O)N([Si](C)(C)C)[Si](C)(C)C | 1928.9 | Semi standard non polar | 33892256 | | Ureidosuccinic acid,3TMS,isomer #5 | C[Si](C)(C)OC(=O)[C@H](CC(=O)O)NC(=O)N([Si](C)(C)C)[Si](C)(C)C | 1848.1 | Standard non polar | 33892256 | | Ureidosuccinic acid,3TMS,isomer #5 | C[Si](C)(C)OC(=O)[C@H](CC(=O)O)NC(=O)N([Si](C)(C)C)[Si](C)(C)C | 2536.6 | Standard polar | 33892256 | | Ureidosuccinic acid,3TMS,isomer #6 | C[Si](C)(C)NC(=O)N([C@@H](CC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C | 1880.2 | Semi standard non polar | 33892256 | | Ureidosuccinic acid,3TMS,isomer #6 | C[Si](C)(C)NC(=O)N([C@@H](CC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C | 1850.1 | Standard non polar | 33892256 | | Ureidosuccinic acid,3TMS,isomer #6 | C[Si](C)(C)NC(=O)N([C@@H](CC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C | 2448.5 | Standard polar | 33892256 | | Ureidosuccinic acid,3TMS,isomer #7 | C[Si](C)(C)N(C(=O)N([Si](C)(C)C)[Si](C)(C)C)[C@@H](CC(=O)O)C(=O)O | 1974.3 | Semi standard non polar | 33892256 | | Ureidosuccinic acid,3TMS,isomer #7 | C[Si](C)(C)N(C(=O)N([Si](C)(C)C)[Si](C)(C)C)[C@@H](CC(=O)O)C(=O)O | 1982.1 | Standard non polar | 33892256 | | Ureidosuccinic acid,3TMS,isomer #7 | C[Si](C)(C)N(C(=O)N([Si](C)(C)C)[Si](C)(C)C)[C@@H](CC(=O)O)C(=O)O | 2474.0 | Standard polar | 33892256 | | Ureidosuccinic acid,4TMS,isomer #1 | C[Si](C)(C)OC(=O)C[C@H](NC(=O)N([Si](C)(C)C)[Si](C)(C)C)C(=O)O[Si](C)(C)C | 1983.5 | Semi standard non polar | 33892256 | | Ureidosuccinic acid,4TMS,isomer #1 | C[Si](C)(C)OC(=O)C[C@H](NC(=O)N([Si](C)(C)C)[Si](C)(C)C)C(=O)O[Si](C)(C)C | 1869.0 | Standard non polar | 33892256 | | Ureidosuccinic acid,4TMS,isomer #1 | C[Si](C)(C)OC(=O)C[C@H](NC(=O)N([Si](C)(C)C)[Si](C)(C)C)C(=O)O[Si](C)(C)C | 2332.8 | Standard polar | 33892256 | | Ureidosuccinic acid,4TMS,isomer #2 | C[Si](C)(C)NC(=O)N([C@@H](CC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C | 1931.1 | Semi standard non polar | 33892256 | | Ureidosuccinic acid,4TMS,isomer #2 | C[Si](C)(C)NC(=O)N([C@@H](CC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C | 1851.7 | Standard non polar | 33892256 | | Ureidosuccinic acid,4TMS,isomer #2 | C[Si](C)(C)NC(=O)N([C@@H](CC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C | 2240.3 | Standard polar | 33892256 | | Ureidosuccinic acid,4TMS,isomer #3 | C[Si](C)(C)OC(=O)C[C@@H](C(=O)O)N(C(=O)N([Si](C)(C)C)[Si](C)(C)C)[Si](C)(C)C | 1959.3 | Semi standard non polar | 33892256 | | Ureidosuccinic acid,4TMS,isomer #3 | C[Si](C)(C)OC(=O)C[C@@H](C(=O)O)N(C(=O)N([Si](C)(C)C)[Si](C)(C)C)[Si](C)(C)C | 2020.1 | Standard non polar | 33892256 | | Ureidosuccinic acid,4TMS,isomer #3 | C[Si](C)(C)OC(=O)C[C@@H](C(=O)O)N(C(=O)N([Si](C)(C)C)[Si](C)(C)C)[Si](C)(C)C | 2248.0 | Standard polar | 33892256 | | Ureidosuccinic acid,4TMS,isomer #4 | C[Si](C)(C)OC(=O)[C@H](CC(=O)O)N(C(=O)N([Si](C)(C)C)[Si](C)(C)C)[Si](C)(C)C | 1969.4 | Semi standard non polar | 33892256 | | Ureidosuccinic acid,4TMS,isomer #4 | C[Si](C)(C)OC(=O)[C@H](CC(=O)O)N(C(=O)N([Si](C)(C)C)[Si](C)(C)C)[Si](C)(C)C | 1992.6 | Standard non polar | 33892256 | | Ureidosuccinic acid,4TMS,isomer #4 | C[Si](C)(C)OC(=O)[C@H](CC(=O)O)N(C(=O)N([Si](C)(C)C)[Si](C)(C)C)[Si](C)(C)C | 2173.3 | Standard polar | 33892256 | | Ureidosuccinic acid,5TMS,isomer #1 | C[Si](C)(C)OC(=O)C[C@@H](C(=O)O[Si](C)(C)C)N(C(=O)N([Si](C)(C)C)[Si](C)(C)C)[Si](C)(C)C | 2014.9 | Semi standard non polar | 33892256 | | Ureidosuccinic acid,5TMS,isomer #1 | C[Si](C)(C)OC(=O)C[C@@H](C(=O)O[Si](C)(C)C)N(C(=O)N([Si](C)(C)C)[Si](C)(C)C)[Si](C)(C)C | 2021.7 | Standard non polar | 33892256 | | Ureidosuccinic acid,5TMS,isomer #1 | C[Si](C)(C)OC(=O)C[C@@H](C(=O)O[Si](C)(C)C)N(C(=O)N([Si](C)(C)C)[Si](C)(C)C)[Si](C)(C)C | 2029.1 | Standard polar | 33892256 | | Ureidosuccinic acid,1TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)C[C@H](NC(N)=O)C(=O)O | 2060.7 | Semi standard non polar | 33892256 | | Ureidosuccinic acid,1TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OC(=O)[C@H](CC(=O)O)NC(N)=O | 2005.8 | Semi standard non polar | 33892256 | | Ureidosuccinic acid,1TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)NC(=O)N[C@@H](CC(=O)O)C(=O)O | 2094.9 | Semi standard non polar | 33892256 | | Ureidosuccinic acid,1TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)N(C(N)=O)[C@@H](CC(=O)O)C(=O)O | 2075.8 | Semi standard non polar | 33892256 | | Ureidosuccinic acid,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)C[C@H](NC(N)=O)C(=O)O[Si](C)(C)C(C)(C)C | 2318.0 | Semi standard non polar | 33892256 | | Ureidosuccinic acid,2TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)NC(=O)N[C@@H](CC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O | 2411.0 | Semi standard non polar | 33892256 | | Ureidosuccinic acid,2TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC(=O)C[C@@H](C(=O)O)N(C(N)=O)[Si](C)(C)C(C)(C)C | 2319.7 | Semi standard non polar | 33892256 | | Ureidosuccinic acid,2TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)NC(=O)N[C@@H](CC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C | 2344.6 | Semi standard non polar | 33892256 | | Ureidosuccinic acid,2TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)OC(=O)[C@H](CC(=O)O)N(C(N)=O)[Si](C)(C)C(C)(C)C | 2266.7 | Semi standard non polar | 33892256 | | Ureidosuccinic acid,2TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)N(C(=O)N[C@@H](CC(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C | 2363.8 | Semi standard non polar | 33892256 | | Ureidosuccinic acid,2TBDMS,isomer #7 | CC(C)(C)[Si](C)(C)NC(=O)N([C@@H](CC(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C | 2322.2 | Semi standard non polar | 33892256 | | Ureidosuccinic acid,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)NC(=O)N[C@@H](CC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C | 2567.5 | Semi standard non polar | 33892256 | | Ureidosuccinic acid,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)NC(=O)N[C@@H](CC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C | 2304.1 | Standard non polar | 33892256 | | Ureidosuccinic acid,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)NC(=O)N[C@@H](CC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C | 2724.9 | Standard polar | 33892256 | | Ureidosuccinic acid,3TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OC(=O)C[C@@H](C(=O)O[Si](C)(C)C(C)(C)C)N(C(N)=O)[Si](C)(C)C(C)(C)C | 2461.9 | Semi standard non polar | 33892256 | | Ureidosuccinic acid,3TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OC(=O)C[C@@H](C(=O)O[Si](C)(C)C(C)(C)C)N(C(N)=O)[Si](C)(C)C(C)(C)C | 2344.5 | Standard non polar | 33892256 | | Ureidosuccinic acid,3TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OC(=O)C[C@@H](C(=O)O[Si](C)(C)C(C)(C)C)N(C(N)=O)[Si](C)(C)C(C)(C)C | 2934.5 | Standard polar | 33892256 | | Ureidosuccinic acid,3TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC(=O)C[C@H](NC(=O)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)C(=O)O | 2593.7 | Semi standard non polar | 33892256 | | Ureidosuccinic acid,3TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC(=O)C[C@H](NC(=O)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)C(=O)O | 2433.0 | Standard non polar | 33892256 | | Ureidosuccinic acid,3TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC(=O)C[C@H](NC(=O)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)C(=O)O | 2759.7 | Standard polar | 33892256 | | Ureidosuccinic acid,3TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)NC(=O)N([C@@H](CC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)[Si](C)(C)C(C)(C)C | 2552.3 | Semi standard non polar | 33892256 | | Ureidosuccinic acid,3TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)NC(=O)N([C@@H](CC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)[Si](C)(C)C(C)(C)C | 2380.8 | Standard non polar | 33892256 | | Ureidosuccinic acid,3TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)NC(=O)N([C@@H](CC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)[Si](C)(C)C(C)(C)C | 2700.2 | Standard polar | 33892256 | | Ureidosuccinic acid,3TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)OC(=O)[C@H](CC(=O)O)NC(=O)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2565.0 | Semi standard non polar | 33892256 | | Ureidosuccinic acid,3TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)OC(=O)[C@H](CC(=O)O)NC(=O)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2415.7 | Standard non polar | 33892256 | | Ureidosuccinic acid,3TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)OC(=O)[C@H](CC(=O)O)NC(=O)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2685.2 | Standard polar | 33892256 | | Ureidosuccinic acid,3TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)NC(=O)N([C@@H](CC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2539.6 | Semi standard non polar | 33892256 | | Ureidosuccinic acid,3TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)NC(=O)N([C@@H](CC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2366.5 | Standard non polar | 33892256 | | Ureidosuccinic acid,3TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)NC(=O)N([C@@H](CC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2664.1 | Standard polar | 33892256 | | Ureidosuccinic acid,3TBDMS,isomer #7 | CC(C)(C)[Si](C)(C)N(C(=O)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[C@@H](CC(=O)O)C(=O)O | 2577.2 | Semi standard non polar | 33892256 | | Ureidosuccinic acid,3TBDMS,isomer #7 | CC(C)(C)[Si](C)(C)N(C(=O)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[C@@H](CC(=O)O)C(=O)O | 2458.2 | Standard non polar | 33892256 | | Ureidosuccinic acid,3TBDMS,isomer #7 | CC(C)(C)[Si](C)(C)N(C(=O)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[C@@H](CC(=O)O)C(=O)O | 2652.3 | Standard polar | 33892256 | | Ureidosuccinic acid,4TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)C[C@H](NC(=O)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C | 2782.0 | Semi standard non polar | 33892256 | | Ureidosuccinic acid,4TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)C[C@H](NC(=O)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C | 2605.2 | Standard non polar | 33892256 | | Ureidosuccinic acid,4TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)C[C@H](NC(=O)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C | 2655.5 | Standard polar | 33892256 | | Ureidosuccinic acid,4TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)NC(=O)N([C@@H](CC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2737.5 | Semi standard non polar | 33892256 | | Ureidosuccinic acid,4TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)NC(=O)N([C@@H](CC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2557.7 | Standard non polar | 33892256 | | Ureidosuccinic acid,4TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)NC(=O)N([C@@H](CC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2646.5 | Standard polar | 33892256 | | Ureidosuccinic acid,4TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC(=O)C[C@@H](C(=O)O)N(C(=O)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2817.5 | Semi standard non polar | 33892256 | | Ureidosuccinic acid,4TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC(=O)C[C@@H](C(=O)O)N(C(=O)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2684.2 | Standard non polar | 33892256 | | Ureidosuccinic acid,4TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC(=O)C[C@@H](C(=O)O)N(C(=O)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2624.1 | Standard polar | 33892256 | | Ureidosuccinic acid,4TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)OC(=O)[C@H](CC(=O)O)N(C(=O)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2812.1 | Semi standard non polar | 33892256 | | Ureidosuccinic acid,4TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)OC(=O)[C@H](CC(=O)O)N(C(=O)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2668.6 | Standard non polar | 33892256 | | Ureidosuccinic acid,4TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)OC(=O)[C@H](CC(=O)O)N(C(=O)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2579.4 | Standard polar | 33892256 | | Ureidosuccinic acid,5TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)C[C@@H](C(=O)O[Si](C)(C)C(C)(C)C)N(C(=O)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 3026.6 | Semi standard non polar | 33892256 | | Ureidosuccinic acid,5TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)C[C@@H](C(=O)O[Si](C)(C)C(C)(C)C)N(C(=O)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2882.5 | Standard non polar | 33892256 | | Ureidosuccinic acid,5TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)C[C@@H](C(=O)O[Si](C)(C)C(C)(C)C)N(C(=O)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2593.4 | Standard polar | 33892256 |
|
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental GC-MS | GC-MS Spectrum - Ureidosuccinic acid GC-MS (4 TMS) | splash10-0f89-3940000000-f6b458a8d8c1bb66550a | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Ureidosuccinic acid GC-MS (3 TMS) | splash10-03di-1910000000-8a8dd39c35aa7c99f81a | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Ureidosuccinic acid EI-B (Non-derivatized) | splash10-03di-0920000000-cdbae9a5c80fb0023f8e | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Ureidosuccinic acid GC-MS (Non-derivatized) | splash10-0f89-3940000000-f6b458a8d8c1bb66550a | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Ureidosuccinic acid GC-MS (Non-derivatized) | splash10-03di-1910000000-8a8dd39c35aa7c99f81a | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Ureidosuccinic acid GC-MS (Non-derivatized) - 70eV, Positive | splash10-0016-9600000000-c3f9041273a7b07c96ad | 2016-09-22 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Ureidosuccinic acid GC-MS (2 TMS) - 70eV, Positive | splash10-00di-9661000000-59d277eae56bc52aa7fa | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Ureidosuccinic acid GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Ureidosuccinic acid GC-MS (TMS_1_1) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Ureidosuccinic acid GC-MS (TMS_1_2) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Ureidosuccinic acid GC-MS (TMS_1_3) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Ureidosuccinic acid GC-MS (TMS_1_4) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Ureidosuccinic acid GC-MS (TMS_2_2) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Ureidosuccinic acid GC-MS (TMS_2_3) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Ureidosuccinic acid GC-MS (TMS_2_4) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Ureidosuccinic acid GC-MS (TMS_2_5) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Ureidosuccinic acid GC-MS (TMS_2_6) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Ureidosuccinic acid GC-MS (TMS_2_7) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Ureidosuccinic acid GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Ureidosuccinic acid GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Ureidosuccinic acid GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Ureidosuccinic acid GC-MS (TBDMS_1_4) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Ureidosuccinic acid GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Ureidosuccinic acid GC-MS (TBDMS_2_2) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Ureidosuccinic acid GC-MS (TBDMS_2_3) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental LC-MS/MS | LC-MS/MS Spectrum - Ureidosuccinic acid Quattro_QQQ 10V, Positive-QTOF (Annotated) | splash10-001i-2900000000-8579bc85efea27463b1a | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Ureidosuccinic acid Quattro_QQQ 25V, Positive-QTOF (Annotated) | splash10-00di-9000000000-bfd715a5dec069d32aea | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Ureidosuccinic acid Quattro_QQQ 40V, Positive-QTOF (Annotated) | splash10-00di-9000000000-17769f0943f627a57c64 | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Ureidosuccinic acid LC-ESI-QQ (API3000, Applied Biosystems) 10V, Negative-QTOF | splash10-0059-0900000000-d1e136c596eda61fdad6 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Ureidosuccinic acid LC-ESI-QQ (API3000, Applied Biosystems) 20V, Negative-QTOF | splash10-001i-1900000000-d9c4b6edb79ec7db500c | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Ureidosuccinic acid LC-ESI-QQ (API3000, Applied Biosystems) 30V, Negative-QTOF | splash10-000i-9500000000-8a0f02bbd32fdd0af067 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Ureidosuccinic acid LC-ESI-QQ (API3000, Applied Biosystems) 40V, Negative-QTOF | splash10-000i-9100000000-c1e7be64cc9ef25c1291 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Ureidosuccinic acid LC-ESI-QQ (API3000, Applied Biosystems) 50V, Negative-QTOF | splash10-0006-9000000000-d90115a5ff29ff776135 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Ureidosuccinic acid LC-ESI-QQ , negative-QTOF | splash10-0059-0900000000-d1e136c596eda61fdad6 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Ureidosuccinic acid LC-ESI-QQ , negative-QTOF | splash10-001i-1900000000-d9c4b6edb79ec7db500c | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Ureidosuccinic acid LC-ESI-QQ , negative-QTOF | splash10-000i-9500000000-ec02387ba0107a9f05cb | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Ureidosuccinic acid LC-ESI-QQ , negative-QTOF | splash10-000i-9100000000-c1e7be64cc9ef25c1291 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Ureidosuccinic acid LC-ESI-QQ , negative-QTOF | splash10-0006-9000000000-d90115a5ff29ff776135 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Ureidosuccinic acid 10V, Negative-QTOF | splash10-001r-3900000000-6d42697f12c0ea7cb5ca | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Ureidosuccinic acid 20V, Negative-QTOF | splash10-000i-9600000000-29b7d3e00a4f3a870861 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Ureidosuccinic acid 40V, Negative-QTOF | splash10-004r-9300000000-e33705943fe244cfa023 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Ureidosuccinic acid 30V, Negative-QTOF | splash10-000i-9400000000-89114ed1862c7416a016 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Ureidosuccinic acid 10V, Positive-QTOF | splash10-057i-1900000000-e642a1d611b0cd263a5b | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Ureidosuccinic acid 20V, Positive-QTOF | splash10-0310-8900000000-0ed6a7e85a30fafb07b2 | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Ureidosuccinic acid 40V, Positive-QTOF | splash10-000l-9100000000-48fee59912455e39938a | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Ureidosuccinic acid 10V, Negative-QTOF | splash10-001l-6900000000-9bae4efc52500be1a047 | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Ureidosuccinic acid 20V, Negative-QTOF | splash10-001c-9400000000-0fc35cb79f71cc7fbdf1 | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Ureidosuccinic acid 40V, Negative-QTOF | splash10-0006-9000000000-27e270ecc918b43d4a41 | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Ureidosuccinic acid 10V, Negative-QTOF | splash10-02a9-7900000000-58b14b371a249024f2f7 | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Ureidosuccinic acid 20V, Negative-QTOF | splash10-000i-9300000000-1c5b264ddc49bbfb87f1 | 2021-09-24 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Experimental 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, experimental) | 2021-10-10 | Wishart Lab | View Spectrum | | Experimental 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, experimental) | 2021-10-10 | Wishart Lab | View Spectrum | | Experimental 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | 2012-12-05 | Wishart Lab | View Spectrum |
IR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted IR Spectrum | IR Ion Spectrum (Quadrupole Ion Trap, ESI+, Adduct: [M+H]+) | 2022-02-11 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Quadrupole Ion Trap, ESI-, Adduct: [M-H]-) | 2022-02-11 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Quadrupole Ion Trap, ESI+, Adduct: [M+Na]+) | 2022-02-11 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M-H]-) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+H]+) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+Na]+) | 2023-02-03 | FELIX lab | View Spectrum |
|
|---|
| General References | - Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM: Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009 Feb 12;457(7231):910-4. doi: 10.1038/nature07762. [PubMed:19212411 ]
- Ohdoi C, Nyhan WL, Kuhara T: Chemical diagnosis of Lesch-Nyhan syndrome using gas chromatography-mass spectrometry detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2003 Jul 15;792(1):123-30. [PubMed:12829005 ]
- van Kuilenburg AB, van Lenthe H, Loffler M, van Gennip AH: Analysis of pyrimidine synthesis "de novo" intermediates in urine and dried urine filter- paper strips with HPLC-electrospray tandem mass spectrometry. Clin Chem. 2004 Nov;50(11):2117-24. Epub 2004 Sep 16. [PubMed:15375016 ]
- Shen C, Sun Z, Chen D, Su X, Jiang J, Li G, Lin B, Yan J: Developing urinary metabolomic signatures as early bladder cancer diagnostic markers. OMICS. 2015 Jan;19(1):1-11. doi: 10.1089/omi.2014.0116. [PubMed:25562196 ]
|
|---|