| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected and Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2023-02-21 17:15:14 UTC |
|---|
| HMDB ID | HMDB0000854 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Formiminoglutamic acid |
|---|
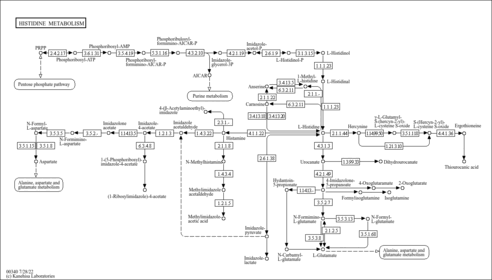
| Description | Formiminoglutamic acid, also known as N-formimino-L-glutamate or formimino-glu, belongs to the class of organic compounds known as glutamic acid and derivatives. Glutamic acid and derivatives are compounds containing glutamic acid or a derivative thereof resulting from reaction of glutamic acid at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. Formiminoglutamic acid exists in all living organisms, ranging from bacteria to humans. In humans, formiminoglutamic acid is involved in the histidine metabolism pathway. Formiminoglutamic acid has been detected, but not quantified in, a few different foods, such as anatidaes (Anatidae), chickens (Gallus gallus), and domestic pigs (Sus scrofa domestica). This could make formiminoglutamic acid a potential biomarker for the consumption of these foods. Formiminoglutamic acid is a primary metabolite. Primary metabolites are metabolically or physiologically essential metabolites. They are directly involved in an organism’s growth, development or reproduction. Based on a literature review a significant number of articles have been published on Formiminoglutamic acid. |
|---|
| Structure | OC(=O)CC[C@H](NC=N)C(O)=O InChI=1S/C6H10N2O4/c7-3-8-4(6(11)12)1-2-5(9)10/h3-4H,1-2H2,(H2,7,8)(H,9,10)(H,11,12)/t4-/m0/s1 |
|---|
| Synonyms | | Value | Source |
|---|
| N-Formimidoyl-L-glutamate | ChEBI | | N-Formimino-L-glutamate | ChEBI | | N-Formimidoyl-L-glutamic acid | Generator | | N-Formimino-L-glutamic acid | Generator | | Formiminoglutamate | Generator | | Formimino-glu | HMDB | | Formimino-L-glutamate | HMDB | | Formimino-L-glutamic acid | HMDB | | N-(Iminomethyl)-L-glutamic acid | HMDB | | N-Formimidoyl-glutamic acid | HMDB | | N-Formimino-glutamate | HMDB | | Acid, formiminoglutamic | HMDB | | FIGLU | HMDB |
|
|---|
| Chemical Formula | C6H10N2O4 |
|---|
| Average Molecular Weight | 174.1546 |
|---|
| Monoisotopic Molecular Weight | 174.064056818 |
|---|
| IUPAC Name | (2S)-2-methanimidamidopentanedioic acid |
|---|
| Traditional Name | N-formimino-L-glutamate |
|---|
| CAS Registry Number | 816-90-0 |
|---|
| SMILES | OC(=O)CC[C@H](NC=N)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C6H10N2O4/c7-3-8-4(6(11)12)1-2-5(9)10/h3-4H,1-2H2,(H2,7,8)(H,9,10)(H,11,12)/t4-/m0/s1 |
|---|
| InChI Key | NRXIKWMTVXPVEF-BYPYZUCNSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as glutamic acid and derivatives. Glutamic acid and derivatives are compounds containing glutamic acid or a derivative thereof resulting from reaction of glutamic acid at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Glutamic acid and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Glutamic acid or derivatives
- Dicarboxylic acid or derivatives
- Fatty acid
- Amidine
- Carboxylic acid amidine
- Carboxylic acid
- Carboximidamide
- Propargyl-type 1,3-dipolar organic compound
- Organic 1,3-dipolar compound
- Formamidine
- Organooxygen compound
- Carbonyl group
- Organic nitrogen compound
- Organonitrogen compound
- Hydrocarbon derivative
- Organic oxygen compound
- Organic oxide
- Organopnictogen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Not Available |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| Formiminoglutamic acid,1TMS,isomer #1 | C[Si](C)(C)OC(=O)CC[C@H](NC=N)C(=O)O | 1801.7 | Semi standard non polar | 33892256 | | Formiminoglutamic acid,1TMS,isomer #2 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC=N | 1762.5 | Semi standard non polar | 33892256 | | Formiminoglutamic acid,1TMS,isomer #3 | C[Si](C)(C)N(C=N)[C@@H](CCC(=O)O)C(=O)O | 1875.6 | Semi standard non polar | 33892256 | | Formiminoglutamic acid,1TMS,isomer #4 | C[Si](C)(C)N=CN[C@@H](CCC(=O)O)C(=O)O | 1813.9 | Semi standard non polar | 33892256 | | Formiminoglutamic acid,2TMS,isomer #1 | C[Si](C)(C)OC(=O)CC[C@H](NC=N)C(=O)O[Si](C)(C)C | 1821.6 | Semi standard non polar | 33892256 | | Formiminoglutamic acid,2TMS,isomer #2 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O)N(C=N)[Si](C)(C)C | 1904.4 | Semi standard non polar | 33892256 | | Formiminoglutamic acid,2TMS,isomer #3 | C[Si](C)(C)N=CN[C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O | 1818.3 | Semi standard non polar | 33892256 | | Formiminoglutamic acid,2TMS,isomer #4 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)N(C=N)[Si](C)(C)C | 1903.3 | Semi standard non polar | 33892256 | | Formiminoglutamic acid,2TMS,isomer #5 | C[Si](C)(C)N=CN[C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C | 1815.7 | Semi standard non polar | 33892256 | | Formiminoglutamic acid,2TMS,isomer #6 | C[Si](C)(C)N=CN([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C | 1910.8 | Semi standard non polar | 33892256 | | Formiminoglutamic acid,3TMS,isomer #1 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O[Si](C)(C)C)N(C=N)[Si](C)(C)C | 1911.4 | Semi standard non polar | 33892256 | | Formiminoglutamic acid,3TMS,isomer #1 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O[Si](C)(C)C)N(C=N)[Si](C)(C)C | 1840.3 | Standard non polar | 33892256 | | Formiminoglutamic acid,3TMS,isomer #1 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O[Si](C)(C)C)N(C=N)[Si](C)(C)C | 2412.8 | Standard polar | 33892256 | | Formiminoglutamic acid,3TMS,isomer #2 | C[Si](C)(C)N=CN[C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C | 1849.1 | Semi standard non polar | 33892256 | | Formiminoglutamic acid,3TMS,isomer #2 | C[Si](C)(C)N=CN[C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C | 1683.3 | Standard non polar | 33892256 | | Formiminoglutamic acid,3TMS,isomer #2 | C[Si](C)(C)N=CN[C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C | 2671.5 | Standard polar | 33892256 | | Formiminoglutamic acid,3TMS,isomer #3 | C[Si](C)(C)N=CN([C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C | 1901.7 | Semi standard non polar | 33892256 | | Formiminoglutamic acid,3TMS,isomer #3 | C[Si](C)(C)N=CN([C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C | 1879.7 | Standard non polar | 33892256 | | Formiminoglutamic acid,3TMS,isomer #3 | C[Si](C)(C)N=CN([C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C | 2487.8 | Standard polar | 33892256 | | Formiminoglutamic acid,3TMS,isomer #4 | C[Si](C)(C)N=CN([C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C | 1920.8 | Semi standard non polar | 33892256 | | Formiminoglutamic acid,3TMS,isomer #4 | C[Si](C)(C)N=CN([C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C | 1855.2 | Standard non polar | 33892256 | | Formiminoglutamic acid,3TMS,isomer #4 | C[Si](C)(C)N=CN([C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C | 2473.5 | Standard polar | 33892256 | | Formiminoglutamic acid,4TMS,isomer #1 | C[Si](C)(C)N=CN([C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C | 1931.2 | Semi standard non polar | 33892256 | | Formiminoglutamic acid,4TMS,isomer #1 | C[Si](C)(C)N=CN([C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C | 1860.7 | Standard non polar | 33892256 | | Formiminoglutamic acid,4TMS,isomer #1 | C[Si](C)(C)N=CN([C@@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C | 2236.6 | Standard polar | 33892256 | | Formiminoglutamic acid,1TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@H](NC=N)C(=O)O | 2054.0 | Semi standard non polar | 33892256 | | Formiminoglutamic acid,1TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC=N | 2029.1 | Semi standard non polar | 33892256 | | Formiminoglutamic acid,1TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)N(C=N)[C@@H](CCC(=O)O)C(=O)O | 2098.6 | Semi standard non polar | 33892256 | | Formiminoglutamic acid,1TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)N=CN[C@@H](CCC(=O)O)C(=O)O | 2060.5 | Semi standard non polar | 33892256 | | Formiminoglutamic acid,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@H](NC=N)C(=O)O[Si](C)(C)C(C)(C)C | 2253.9 | Semi standard non polar | 33892256 | | Formiminoglutamic acid,2TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@@H](C(=O)O)N(C=N)[Si](C)(C)C(C)(C)C | 2369.1 | Semi standard non polar | 33892256 | | Formiminoglutamic acid,2TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)N=CN[C@@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O | 2268.2 | Semi standard non polar | 33892256 | | Formiminoglutamic acid,2TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)OC(=O)[C@H](CCC(=O)O)N(C=N)[Si](C)(C)C(C)(C)C | 2348.3 | Semi standard non polar | 33892256 | | Formiminoglutamic acid,2TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)N=CN[C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C | 2246.1 | Semi standard non polar | 33892256 | | Formiminoglutamic acid,2TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)N=CN([C@@H](CCC(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C | 2309.9 | Semi standard non polar | 33892256 | | Formiminoglutamic acid,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@@H](C(=O)O[Si](C)(C)C(C)(C)C)N(C=N)[Si](C)(C)C(C)(C)C | 2544.5 | Semi standard non polar | 33892256 | | Formiminoglutamic acid,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@@H](C(=O)O[Si](C)(C)C(C)(C)C)N(C=N)[Si](C)(C)C(C)(C)C | 2460.0 | Standard non polar | 33892256 | | Formiminoglutamic acid,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@@H](C(=O)O[Si](C)(C)C(C)(C)C)N(C=N)[Si](C)(C)C(C)(C)C | 2624.8 | Standard polar | 33892256 | | Formiminoglutamic acid,3TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)N=CN[C@@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C | 2449.9 | Semi standard non polar | 33892256 | | Formiminoglutamic acid,3TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)N=CN[C@@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C | 2280.4 | Standard non polar | 33892256 | | Formiminoglutamic acid,3TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)N=CN[C@@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C | 2721.0 | Standard polar | 33892256 | | Formiminoglutamic acid,3TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)N=CN([C@@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)[Si](C)(C)C(C)(C)C | 2539.5 | Semi standard non polar | 33892256 | | Formiminoglutamic acid,3TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)N=CN([C@@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)[Si](C)(C)C(C)(C)C | 2379.9 | Standard non polar | 33892256 | | Formiminoglutamic acid,3TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)N=CN([C@@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)[Si](C)(C)C(C)(C)C | 2671.2 | Standard polar | 33892256 | | Formiminoglutamic acid,3TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)N=CN([C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2535.2 | Semi standard non polar | 33892256 | | Formiminoglutamic acid,3TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)N=CN([C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2366.3 | Standard non polar | 33892256 | | Formiminoglutamic acid,3TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)N=CN([C@@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2662.3 | Standard polar | 33892256 | | Formiminoglutamic acid,4TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)N=CN([C@@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2745.5 | Semi standard non polar | 33892256 | | Formiminoglutamic acid,4TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)N=CN([C@@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2551.9 | Standard non polar | 33892256 | | Formiminoglutamic acid,4TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)N=CN([C@@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2588.2 | Standard polar | 33892256 |
|
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - Formiminoglutamic acid GC-MS (Non-derivatized) - 70eV, Positive | splash10-056u-9400000000-6cceb3db80c1c51b5798 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Formiminoglutamic acid GC-MS (2 TMS) - 70eV, Positive | splash10-0fg9-9230000000-65123e05e539b5af2388 | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Formiminoglutamic acid GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Formiminoglutamic acid GC-MS (TMS_1_1) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Formiminoglutamic acid GC-MS (TMS_1_2) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Formiminoglutamic acid GC-MS (TMS_1_3) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Formiminoglutamic acid GC-MS (TMS_1_4) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Formiminoglutamic acid GC-MS (TMS_2_2) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Formiminoglutamic acid GC-MS (TMS_2_3) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Formiminoglutamic acid GC-MS (TMS_2_4) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Formiminoglutamic acid GC-MS (TMS_2_5) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Formiminoglutamic acid GC-MS (TMS_2_6) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Formiminoglutamic acid GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Formiminoglutamic acid GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Formiminoglutamic acid GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Formiminoglutamic acid GC-MS (TBDMS_1_4) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Formiminoglutamic acid GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Formiminoglutamic acid GC-MS (TBDMS_2_2) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Formiminoglutamic acid GC-MS (TBDMS_2_3) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Formiminoglutamic acid GC-MS (TBDMS_2_4) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Formiminoglutamic acid GC-MS (TBDMS_2_5) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Formiminoglutamic acid GC-MS (TBDMS_2_6) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Formiminoglutamic acid 10V, Positive-QTOF | splash10-056r-0900000000-92c0f47c419298fb78a2 | 2016-08-03 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Formiminoglutamic acid 20V, Positive-QTOF | splash10-004j-5900000000-8b915dd10756167a4003 | 2016-08-03 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Formiminoglutamic acid 40V, Positive-QTOF | splash10-0012-9100000000-313b0983e834934e5682 | 2016-08-03 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Formiminoglutamic acid 10V, Negative-QTOF | splash10-00fr-1900000000-b264a846efb94105ac49 | 2016-08-04 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Formiminoglutamic acid 20V, Negative-QTOF | splash10-0f97-5900000000-2f4ec838a8ef46f9428d | 2016-08-04 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Formiminoglutamic acid 40V, Negative-QTOF | splash10-002f-9200000000-a518077a6ec2f6a6c6c5 | 2016-08-04 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Formiminoglutamic acid 10V, Positive-QTOF | splash10-004i-0900000000-e21825a4085307dba501 | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Formiminoglutamic acid 20V, Positive-QTOF | splash10-02d0-7900000000-39bddd8ed9efae999a7c | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Formiminoglutamic acid 40V, Positive-QTOF | splash10-0015-9000000000-8230ed13a5a7ee4c82a6 | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Formiminoglutamic acid 10V, Negative-QTOF | splash10-00b9-0900000000-d26547339916cbc25d34 | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Formiminoglutamic acid 20V, Negative-QTOF | splash10-0773-9700000000-d2b6c121d8ab7a8500b7 | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Formiminoglutamic acid 40V, Negative-QTOF | splash10-0006-9100000000-abb21e9862307446d7ec | 2021-09-25 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum |
IR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M-H]-) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+H]+) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+Na]+) | 2023-02-03 | FELIX lab | View Spectrum |
|
|---|
| General References | - Hilton JF, Christensen KE, Watkins D, Raby BA, Renaud Y, de la Luna S, Estivill X, MacKenzie RE, Hudson TJ, Rosenblatt DS: The molecular basis of glutamate formiminotransferase deficiency. Hum Mutat. 2003 Jul;22(1):67-73. [PubMed:12815595 ]
- Haurani FI, Hall CA, Rubin R: Megaloblastic anemia as a result of an abnormal transcobalamin II (Cardeza). J Clin Invest. 1979 Nov;64(5):1253-9. [PubMed:500809 ]
- Perry TL, Applegarth DA, Evans ME, Hansen S, Jellum E: Metabolic studies of a family with massive formiminoglutamic aciduria. Pediatr Res. 1975 Mar;9(3):117-22. [PubMed:235753 ]
- Verhoeven NM, Wanders RJ, Poll-The BT, Saudubray JM, Jakobs C: The metabolism of phytanic acid and pristanic acid in man: a review. J Inherit Metab Dis. 1998 Oct;21(7):697-728. [PubMed:9819701 ]
|
|---|