| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected and Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2022-03-07 02:49:07 UTC |
|---|
| HMDB ID | HMDB0000951 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Taurochenodesoxycholic acid |
|---|
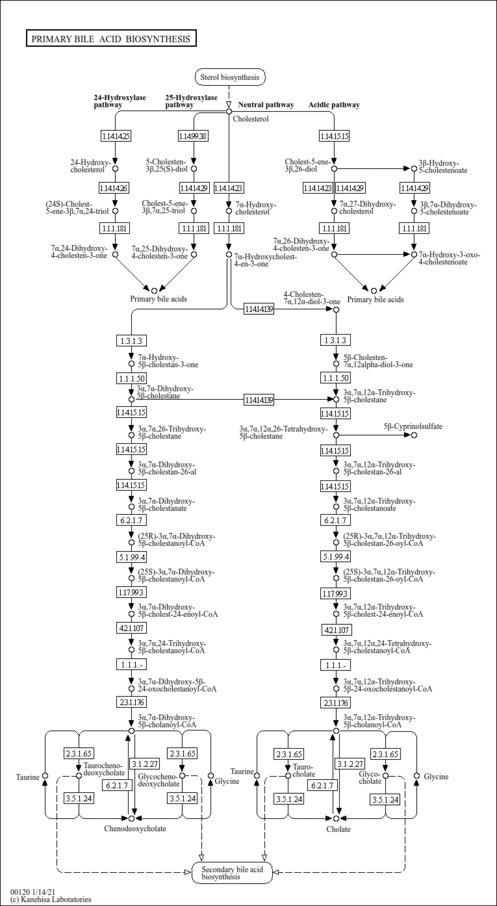
| Description | Taurochenodesoxycholic acid is a bile acid formed in the liver by conjugation of chenodeoxycholate with taurine, usually as the sodium salt. Bile acids are steroid acids found predominantly in the bile of mammals. The distinction between different bile acids is minute, depending only on the presence or absence of hydroxyl groups on positions 3, 7, and 12. Bile acids are physiological detergents that facilitate excretion, absorption, and transport of fats and sterols in the intestine and liver. Bile acids are also steroidal amphipathic molecules derived from the catabolism of cholesterol. They modulate bile flow and lipid secretion, are essential for the absorption of dietary fats and vitamins, and have been implicated in the regulation of all the key enzymes involved in cholesterol homeostasis. Bile acids recirculate through the liver, bile ducts, small intestine and portal vein to form an enterohepatic circuit. They exist as anions at physiological pH and, consequently, require a carrier for transport across the membranes of the enterohepatic tissues. The unique detergent properties of bile acids are essential for the digestion and intestinal absorption of hydrophobic nutrients. Bile acids have potent toxic properties (e.g. membrane disruption) and there are a plethora of mechanisms to limit their accumulation in blood and tissues (PMID: 11316487 , 16037564 , 12576301 , 11907135 ). Taurochenodesoxycholic acid has been found to be a microbial metabolite. |
|---|
| Structure | [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(=O)NCCS(O)(=O)=O InChI=1S/C26H45NO6S/c1-16(4-7-23(30)27-12-13-34(31,32)33)19-5-6-20-24-21(9-11-26(19,20)3)25(2)10-8-18(28)14-17(25)15-22(24)29/h16-22,24,28-29H,4-15H2,1-3H3,(H,27,30)(H,31,32,33)/t16-,17+,18-,19-,20+,21+,22-,24+,25+,26-/m1/s1 |
|---|
| Synonyms | | Value | Source |
|---|
| Chenodeoxycholoyltaurine | ChEBI | | Taurine chenodeoxycholate | ChEBI | | Taurochenodeoxycholate | ChEBI | | Taurochenodeoxycholic acid | Kegg | | Taurine chenodeoxycholic acid | Generator | | Taurochenodesoxycholate | Generator | | 12-Deoxycholyltaurine | HMDB | | 12-Desoxycholyltaurine | HMDB | | 3a,7a-Dihydroxy-N-(2-sulfoethyl)-5b-cholan-24-amide | HMDB | | Chenodeoxycholyltaurine | HMDB | | Chenyltaurine | HMDB | | N-(3a,7a-Dihydroxy-5b-cholan-24-oyl)-taurine | HMDB | | Acid, taurochenodeoxycholic | HMDB | | Chenodeoxycholate, taurine | HMDB |
|
|---|
| Chemical Formula | C26H45NO6S |
|---|
| Average Molecular Weight | 499.704 |
|---|
| Monoisotopic Molecular Weight | 499.296758867 |
|---|
| IUPAC Name | 2-[(4R)-4-[(1S,2S,5R,7S,9R,10R,11S,14R,15R)-5,9-dihydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadecan-14-yl]pentanamido]ethane-1-sulfonic acid |
|---|
| Traditional Name | 2-[(4R)-4-[(1S,2S,5R,7S,9R,10R,11S,14R,15R)-5,9-dihydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadecan-14-yl]pentanamido]ethanesulfonic acid |
|---|
| CAS Registry Number | 516-35-8 |
|---|
| SMILES | [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(=O)NCCS(O)(=O)=O |
|---|
| InChI Identifier | InChI=1S/C26H45NO6S/c1-16(4-7-23(30)27-12-13-34(31,32)33)19-5-6-20-24-21(9-11-26(19,20)3)25(2)10-8-18(28)14-17(25)15-22(24)29/h16-22,24,28-29H,4-15H2,1-3H3,(H,27,30)(H,31,32,33)/t16-,17+,18-,19-,20+,21+,22-,24+,25+,26-/m1/s1 |
|---|
| InChI Key | BHTRKEVKTKCXOH-BJLOMENOSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as taurinated bile acids and derivatives. These are bile acid derivatives containing a taurine conjugated to the bile acid moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Bile acids, alcohols and derivatives |
|---|
| Direct Parent | Taurinated bile acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Taurinated bile acid
- Dihydroxy bile acid, alcohol, or derivatives
- Hydroxy bile acid, alcohol, or derivatives
- 3-hydroxysteroid
- Hydroxysteroid
- 3-alpha-hydroxysteroid
- 7-hydroxysteroid
- Fatty acyl
- Fatty amide
- N-acyl-amine
- Alkanesulfonic acid
- Cyclic alcohol
- Organic sulfonic acid or derivatives
- Organosulfonic acid or derivatives
- Organosulfonic acid
- Sulfonyl
- Secondary alcohol
- Secondary carboxylic acid amide
- Carboxamide group
- Carboxylic acid derivative
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Alcohol
- Organic oxygen compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Carbonyl group
- Organic oxide
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Experimental Collision Cross Sections |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| Taurochenodesoxycholic acid,1TMS,isomer #1 | C[C@H](CCC(=O)NCCS(=O)(=O)O)[C@H]1CC[C@H]2[C@H]3[C@H](CC[C@@]21C)[C@@]1(C)CC[C@@H](O)C[C@H]1C[C@H]3O[Si](C)(C)C | 4160.5 | Semi standard non polar | 33892256 | | Taurochenodesoxycholic acid,1TMS,isomer #2 | C[C@H](CCC(=O)NCCS(=O)(=O)O)[C@H]1CC[C@H]2[C@H]3[C@H](CC[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C)C[C@H]1C[C@H]3O | 4274.3 | Semi standard non polar | 33892256 | | Taurochenodesoxycholic acid,1TMS,isomer #3 | C[C@H](CCC(=O)NCCS(=O)(=O)O[Si](C)(C)C)[C@H]1CC[C@H]2[C@H]3[C@H](CC[C@@]21C)[C@@]1(C)CC[C@@H](O)C[C@H]1C[C@H]3O | 4333.3 | Semi standard non polar | 33892256 | | Taurochenodesoxycholic acid,1TMS,isomer #4 | C[C@H](CCC(=O)N(CCS(=O)(=O)O)[Si](C)(C)C)[C@H]1CC[C@H]2[C@H]3[C@H](CC[C@@]21C)[C@@]1(C)CC[C@@H](O)C[C@H]1C[C@H]3O | 4263.8 | Semi standard non polar | 33892256 | | Taurochenodesoxycholic acid,2TMS,isomer #1 | C[C@H](CCC(=O)NCCS(=O)(=O)O)[C@H]1CC[C@H]2[C@H]3[C@H](CC[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C)C[C@H]1C[C@H]3O[Si](C)(C)C | 4115.9 | Semi standard non polar | 33892256 | | Taurochenodesoxycholic acid,2TMS,isomer #2 | C[C@H](CCC(=O)NCCS(=O)(=O)O[Si](C)(C)C)[C@H]1CC[C@H]2[C@H]3[C@H](CC[C@@]21C)[C@@]1(C)CC[C@@H](O)C[C@H]1C[C@H]3O[Si](C)(C)C | 4193.1 | Semi standard non polar | 33892256 | | Taurochenodesoxycholic acid,2TMS,isomer #3 | C[C@H](CCC(=O)N(CCS(=O)(=O)O)[Si](C)(C)C)[C@H]1CC[C@H]2[C@H]3[C@H](CC[C@@]21C)[C@@]1(C)CC[C@@H](O)C[C@H]1C[C@H]3O[Si](C)(C)C | 4085.7 | Semi standard non polar | 33892256 | | Taurochenodesoxycholic acid,2TMS,isomer #4 | C[C@H](CCC(=O)NCCS(=O)(=O)O[Si](C)(C)C)[C@H]1CC[C@H]2[C@H]3[C@H](CC[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C)C[C@H]1C[C@H]3O | 4329.0 | Semi standard non polar | 33892256 | | Taurochenodesoxycholic acid,2TMS,isomer #5 | C[C@H](CCC(=O)N(CCS(=O)(=O)O)[Si](C)(C)C)[C@H]1CC[C@H]2[C@H]3[C@H](CC[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C)C[C@H]1C[C@H]3O | 4246.2 | Semi standard non polar | 33892256 | | Taurochenodesoxycholic acid,2TMS,isomer #6 | C[C@H](CCC(=O)N(CCS(=O)(=O)O[Si](C)(C)C)[Si](C)(C)C)[C@H]1CC[C@H]2[C@H]3[C@H](CC[C@@]21C)[C@@]1(C)CC[C@@H](O)C[C@H]1C[C@H]3O | 4329.4 | Semi standard non polar | 33892256 | | Taurochenodesoxycholic acid,3TMS,isomer #1 | C[C@H](CCC(=O)NCCS(=O)(=O)O[Si](C)(C)C)[C@H]1CC[C@H]2[C@H]3[C@H](CC[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C)C[C@H]1C[C@H]3O[Si](C)(C)C | 4162.4 | Semi standard non polar | 33892256 | | Taurochenodesoxycholic acid,3TMS,isomer #1 | C[C@H](CCC(=O)NCCS(=O)(=O)O[Si](C)(C)C)[C@H]1CC[C@H]2[C@H]3[C@H](CC[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C)C[C@H]1C[C@H]3O[Si](C)(C)C | 4441.4 | Standard non polar | 33892256 | | Taurochenodesoxycholic acid,3TMS,isomer #1 | C[C@H](CCC(=O)NCCS(=O)(=O)O[Si](C)(C)C)[C@H]1CC[C@H]2[C@H]3[C@H](CC[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C)C[C@H]1C[C@H]3O[Si](C)(C)C | 4880.2 | Standard polar | 33892256 | | Taurochenodesoxycholic acid,3TMS,isomer #2 | C[C@H](CCC(=O)N(CCS(=O)(=O)O)[Si](C)(C)C)[C@H]1CC[C@H]2[C@H]3[C@H](CC[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C)C[C@H]1C[C@H]3O[Si](C)(C)C | 4045.1 | Semi standard non polar | 33892256 | | Taurochenodesoxycholic acid,3TMS,isomer #2 | C[C@H](CCC(=O)N(CCS(=O)(=O)O)[Si](C)(C)C)[C@H]1CC[C@H]2[C@H]3[C@H](CC[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C)C[C@H]1C[C@H]3O[Si](C)(C)C | 4510.2 | Standard non polar | 33892256 | | Taurochenodesoxycholic acid,3TMS,isomer #2 | C[C@H](CCC(=O)N(CCS(=O)(=O)O)[Si](C)(C)C)[C@H]1CC[C@H]2[C@H]3[C@H](CC[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C)C[C@H]1C[C@H]3O[Si](C)(C)C | 4945.1 | Standard polar | 33892256 | | Taurochenodesoxycholic acid,3TMS,isomer #3 | C[C@H](CCC(=O)N(CCS(=O)(=O)O[Si](C)(C)C)[Si](C)(C)C)[C@H]1CC[C@H]2[C@H]3[C@H](CC[C@@]21C)[C@@]1(C)CC[C@@H](O)C[C@H]1C[C@H]3O[Si](C)(C)C | 4142.7 | Semi standard non polar | 33892256 | | Taurochenodesoxycholic acid,3TMS,isomer #3 | C[C@H](CCC(=O)N(CCS(=O)(=O)O[Si](C)(C)C)[Si](C)(C)C)[C@H]1CC[C@H]2[C@H]3[C@H](CC[C@@]21C)[C@@]1(C)CC[C@@H](O)C[C@H]1C[C@H]3O[Si](C)(C)C | 4519.4 | Standard non polar | 33892256 | | Taurochenodesoxycholic acid,3TMS,isomer #3 | C[C@H](CCC(=O)N(CCS(=O)(=O)O[Si](C)(C)C)[Si](C)(C)C)[C@H]1CC[C@H]2[C@H]3[C@H](CC[C@@]21C)[C@@]1(C)CC[C@@H](O)C[C@H]1C[C@H]3O[Si](C)(C)C | 4852.8 | Standard polar | 33892256 | | Taurochenodesoxycholic acid,3TMS,isomer #4 | C[C@H](CCC(=O)N(CCS(=O)(=O)O[Si](C)(C)C)[Si](C)(C)C)[C@H]1CC[C@H]2[C@H]3[C@H](CC[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C)C[C@H]1C[C@H]3O | 4279.2 | Semi standard non polar | 33892256 | | Taurochenodesoxycholic acid,3TMS,isomer #4 | C[C@H](CCC(=O)N(CCS(=O)(=O)O[Si](C)(C)C)[Si](C)(C)C)[C@H]1CC[C@H]2[C@H]3[C@H](CC[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C)C[C@H]1C[C@H]3O | 4507.0 | Standard non polar | 33892256 | | Taurochenodesoxycholic acid,3TMS,isomer #4 | C[C@H](CCC(=O)N(CCS(=O)(=O)O[Si](C)(C)C)[Si](C)(C)C)[C@H]1CC[C@H]2[C@H]3[C@H](CC[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C)C[C@H]1C[C@H]3O | 4830.4 | Standard polar | 33892256 | | Taurochenodesoxycholic acid,4TMS,isomer #1 | C[C@H](CCC(=O)N(CCS(=O)(=O)O[Si](C)(C)C)[Si](C)(C)C)[C@H]1CC[C@H]2[C@H]3[C@H](CC[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C)C[C@H]1C[C@H]3O[Si](C)(C)C | 4130.5 | Semi standard non polar | 33892256 | | Taurochenodesoxycholic acid,4TMS,isomer #1 | C[C@H](CCC(=O)N(CCS(=O)(=O)O[Si](C)(C)C)[Si](C)(C)C)[C@H]1CC[C@H]2[C@H]3[C@H](CC[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C)C[C@H]1C[C@H]3O[Si](C)(C)C | 4653.8 | Standard non polar | 33892256 | | Taurochenodesoxycholic acid,4TMS,isomer #1 | C[C@H](CCC(=O)N(CCS(=O)(=O)O[Si](C)(C)C)[Si](C)(C)C)[C@H]1CC[C@H]2[C@H]3[C@H](CC[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C)C[C@H]1C[C@H]3O[Si](C)(C)C | 4702.8 | Standard polar | 33892256 | | Taurochenodesoxycholic acid,1TBDMS,isomer #1 | C[C@H](CCC(=O)NCCS(=O)(=O)O)[C@H]1CC[C@H]2[C@H]3[C@H](CC[C@@]21C)[C@@]1(C)CC[C@@H](O)C[C@H]1C[C@H]3O[Si](C)(C)C(C)(C)C | 4355.7 | Semi standard non polar | 33892256 | | Taurochenodesoxycholic acid,1TBDMS,isomer #2 | C[C@H](CCC(=O)NCCS(=O)(=O)O)[C@H]1CC[C@H]2[C@H]3[C@H](CC[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C(C)(C)C)C[C@H]1C[C@H]3O | 4473.3 | Semi standard non polar | 33892256 | | Taurochenodesoxycholic acid,1TBDMS,isomer #3 | C[C@H](CCC(=O)NCCS(=O)(=O)O[Si](C)(C)C(C)(C)C)[C@H]1CC[C@H]2[C@H]3[C@H](CC[C@@]21C)[C@@]1(C)CC[C@@H](O)C[C@H]1C[C@H]3O | 4519.7 | Semi standard non polar | 33892256 | | Taurochenodesoxycholic acid,1TBDMS,isomer #4 | C[C@H](CCC(=O)N(CCS(=O)(=O)O)[Si](C)(C)C(C)(C)C)[C@H]1CC[C@H]2[C@H]3[C@H](CC[C@@]21C)[C@@]1(C)CC[C@@H](O)C[C@H]1C[C@H]3O | 4520.9 | Semi standard non polar | 33892256 | | Taurochenodesoxycholic acid,2TBDMS,isomer #1 | C[C@H](CCC(=O)NCCS(=O)(=O)O)[C@H]1CC[C@H]2[C@H]3[C@H](CC[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C(C)(C)C)C[C@H]1C[C@H]3O[Si](C)(C)C(C)(C)C | 4506.3 | Semi standard non polar | 33892256 | | Taurochenodesoxycholic acid,2TBDMS,isomer #2 | C[C@H](CCC(=O)NCCS(=O)(=O)O[Si](C)(C)C(C)(C)C)[C@H]1CC[C@H]2[C@H]3[C@H](CC[C@@]21C)[C@@]1(C)CC[C@@H](O)C[C@H]1C[C@H]3O[Si](C)(C)C(C)(C)C | 4578.1 | Semi standard non polar | 33892256 | | Taurochenodesoxycholic acid,2TBDMS,isomer #3 | C[C@H](CCC(=O)N(CCS(=O)(=O)O)[Si](C)(C)C(C)(C)C)[C@H]1CC[C@H]2[C@H]3[C@H](CC[C@@]21C)[C@@]1(C)CC[C@@H](O)C[C@H]1C[C@H]3O[Si](C)(C)C(C)(C)C | 4544.2 | Semi standard non polar | 33892256 | | Taurochenodesoxycholic acid,2TBDMS,isomer #4 | C[C@H](CCC(=O)NCCS(=O)(=O)O[Si](C)(C)C(C)(C)C)[C@H]1CC[C@H]2[C@H]3[C@H](CC[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C(C)(C)C)C[C@H]1C[C@H]3O | 4738.6 | Semi standard non polar | 33892256 | | Taurochenodesoxycholic acid,2TBDMS,isomer #5 | C[C@H](CCC(=O)N(CCS(=O)(=O)O)[Si](C)(C)C(C)(C)C)[C@H]1CC[C@H]2[C@H]3[C@H](CC[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C(C)(C)C)C[C@H]1C[C@H]3O | 4695.3 | Semi standard non polar | 33892256 | | Taurochenodesoxycholic acid,2TBDMS,isomer #6 | C[C@H](CCC(=O)N(CCS(=O)(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[C@H]1CC[C@H]2[C@H]3[C@H](CC[C@@]21C)[C@@]1(C)CC[C@@H](O)C[C@H]1C[C@H]3O | 4779.1 | Semi standard non polar | 33892256 | | Taurochenodesoxycholic acid,3TBDMS,isomer #1 | C[C@H](CCC(=O)NCCS(=O)(=O)O[Si](C)(C)C(C)(C)C)[C@H]1CC[C@H]2[C@H]3[C@H](CC[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C(C)(C)C)C[C@H]1C[C@H]3O[Si](C)(C)C(C)(C)C | 4745.4 | Semi standard non polar | 33892256 | | Taurochenodesoxycholic acid,3TBDMS,isomer #1 | C[C@H](CCC(=O)NCCS(=O)(=O)O[Si](C)(C)C(C)(C)C)[C@H]1CC[C@H]2[C@H]3[C@H](CC[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C(C)(C)C)C[C@H]1C[C@H]3O[Si](C)(C)C(C)(C)C | 5223.1 | Standard non polar | 33892256 | | Taurochenodesoxycholic acid,3TBDMS,isomer #1 | C[C@H](CCC(=O)NCCS(=O)(=O)O[Si](C)(C)C(C)(C)C)[C@H]1CC[C@H]2[C@H]3[C@H](CC[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C(C)(C)C)C[C@H]1C[C@H]3O[Si](C)(C)C(C)(C)C | 4964.3 | Standard polar | 33892256 | | Taurochenodesoxycholic acid,3TBDMS,isomer #2 | C[C@H](CCC(=O)N(CCS(=O)(=O)O)[Si](C)(C)C(C)(C)C)[C@H]1CC[C@H]2[C@H]3[C@H](CC[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C(C)(C)C)C[C@H]1C[C@H]3O[Si](C)(C)C(C)(C)C | 4705.6 | Semi standard non polar | 33892256 | | Taurochenodesoxycholic acid,3TBDMS,isomer #2 | C[C@H](CCC(=O)N(CCS(=O)(=O)O)[Si](C)(C)C(C)(C)C)[C@H]1CC[C@H]2[C@H]3[C@H](CC[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C(C)(C)C)C[C@H]1C[C@H]3O[Si](C)(C)C(C)(C)C | 5232.2 | Standard non polar | 33892256 | | Taurochenodesoxycholic acid,3TBDMS,isomer #2 | C[C@H](CCC(=O)N(CCS(=O)(=O)O)[Si](C)(C)C(C)(C)C)[C@H]1CC[C@H]2[C@H]3[C@H](CC[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C(C)(C)C)C[C@H]1C[C@H]3O[Si](C)(C)C(C)(C)C | 5033.0 | Standard polar | 33892256 | | Taurochenodesoxycholic acid,3TBDMS,isomer #3 | C[C@H](CCC(=O)N(CCS(=O)(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[C@H]1CC[C@H]2[C@H]3[C@H](CC[C@@]21C)[C@@]1(C)CC[C@@H](O)C[C@H]1C[C@H]3O[Si](C)(C)C(C)(C)C | 4781.2 | Semi standard non polar | 33892256 | | Taurochenodesoxycholic acid,3TBDMS,isomer #3 | C[C@H](CCC(=O)N(CCS(=O)(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[C@H]1CC[C@H]2[C@H]3[C@H](CC[C@@]21C)[C@@]1(C)CC[C@@H](O)C[C@H]1C[C@H]3O[Si](C)(C)C(C)(C)C | 5301.6 | Standard non polar | 33892256 | | Taurochenodesoxycholic acid,3TBDMS,isomer #3 | C[C@H](CCC(=O)N(CCS(=O)(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[C@H]1CC[C@H]2[C@H]3[C@H](CC[C@@]21C)[C@@]1(C)CC[C@@H](O)C[C@H]1C[C@H]3O[Si](C)(C)C(C)(C)C | 4952.0 | Standard polar | 33892256 | | Taurochenodesoxycholic acid,3TBDMS,isomer #4 | C[C@H](CCC(=O)N(CCS(=O)(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[C@H]1CC[C@H]2[C@H]3[C@H](CC[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C(C)(C)C)C[C@H]1C[C@H]3O | 4925.9 | Semi standard non polar | 33892256 | | Taurochenodesoxycholic acid,3TBDMS,isomer #4 | C[C@H](CCC(=O)N(CCS(=O)(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[C@H]1CC[C@H]2[C@H]3[C@H](CC[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C(C)(C)C)C[C@H]1C[C@H]3O | 5282.2 | Standard non polar | 33892256 | | Taurochenodesoxycholic acid,3TBDMS,isomer #4 | C[C@H](CCC(=O)N(CCS(=O)(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[C@H]1CC[C@H]2[C@H]3[C@H](CC[C@@]21C)[C@@]1(C)CC[C@@H](O[Si](C)(C)C(C)(C)C)C[C@H]1C[C@H]3O | 4925.1 | Standard polar | 33892256 |
|
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - Taurochenodesoxycholic acid GC-MS (Non-derivatized) - 70eV, Positive | splash10-053r-0212900000-197acffecf402e598644 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Taurochenodesoxycholic acid GC-MS (2 TMS) - 70eV, Positive | splash10-004i-4214329000-11c176071503161e2cd4 | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Taurochenodesoxycholic acid GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Taurochenodesoxycholic acid GC-MS (TMS_1_1) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Taurochenodesoxycholic acid GC-MS (TMS_1_2) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Taurochenodesoxycholic acid GC-MS (TMS_1_3) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Taurochenodesoxycholic acid GC-MS (TMS_1_4) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Taurochenodesoxycholic acid GC-MS (TMS_2_2) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Taurochenodesoxycholic acid GC-MS (TMS_2_3) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Taurochenodesoxycholic acid GC-MS (TMS_2_4) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Taurochenodesoxycholic acid GC-MS (TMS_2_5) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Taurochenodesoxycholic acid GC-MS (TMS_2_6) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Taurochenodesoxycholic acid GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Taurochenodesoxycholic acid GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Taurochenodesoxycholic acid GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Taurochenodesoxycholic acid GC-MS (TBDMS_1_4) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Taurochenodesoxycholic acid GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Taurochenodesoxycholic acid GC-MS (TBDMS_2_2) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Taurochenodesoxycholic acid GC-MS (TBDMS_2_3) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Taurochenodesoxycholic acid GC-MS (TBDMS_2_4) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Taurochenodesoxycholic acid GC-MS (TBDMS_2_5) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Taurochenodesoxycholic acid GC-MS (TBDMS_2_6) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental LC-MS/MS | LC-MS/MS Spectrum - Taurochenodesoxycholic acid LC-ESI-IT , negative-QTOF | splash10-02ar-0006900000-53366e607f8bd2e50783 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Taurochenodesoxycholic acid 40V, Positive-QTOF | splash10-0og0-1595100000-2488fbc0ad2dab225684 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Taurochenodesoxycholic acid 10V, Positive-QTOF | splash10-01q9-0000920000-d308c77003ce029da322 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Taurochenodesoxycholic acid 20V, Positive-QTOF | splash10-03di-0000900000-4f77a6fb2b33e4e1a35f | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Taurochenodesoxycholic acid 10V, Negative-QTOF | splash10-0002-0000900000-671f53e46ce019da43dc | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Taurochenodesoxycholic acid 40V, Positive-QTOF | splash10-0og0-1595100000-6670e8e89de5e9a0c3a1 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Taurochenodesoxycholic acid 10V, Positive-QTOF | splash10-01q9-0000920000-0b63233ca30c95bee595 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Taurochenodesoxycholic acid 20V, Negative-QTOF | splash10-0002-0000900000-54b7fff092e1f9db6a20 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Taurochenodesoxycholic acid 40V, Negative-QTOF | splash10-0002-0000900000-f978cbaedb898eee1843 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Taurochenodesoxycholic acid 10V, Positive-QTOF | splash10-00e9-0801920000-ecfc97935416325b3ca5 | 2016-08-03 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Taurochenodesoxycholic acid 20V, Positive-QTOF | splash10-00di-2901100000-9a3cf7f2963c041be363 | 2016-08-03 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Taurochenodesoxycholic acid 40V, Positive-QTOF | splash10-006x-8904100000-98cfa2606dbba4ef47a0 | 2016-08-03 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Taurochenodesoxycholic acid 10V, Negative-QTOF | splash10-000t-2001900000-4adf0e40de3812e8d0ba | 2016-08-04 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Taurochenodesoxycholic acid 20V, Negative-QTOF | splash10-001i-6404900000-4d05b6f0352aebf4228d | 2016-08-04 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Taurochenodesoxycholic acid 40V, Negative-QTOF | splash10-001l-9102000000-63892e6e06f1123f8bfc | 2016-08-04 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Taurochenodesoxycholic acid 10V, Positive-QTOF | splash10-0ue9-0000970000-8561365963466c5db093 | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Taurochenodesoxycholic acid 20V, Positive-QTOF | splash10-0bvi-3509710000-451e758e0b96f9fa8ee7 | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Taurochenodesoxycholic acid 40V, Positive-QTOF | splash10-00l5-9463000000-16d35ecb5101cbd28ea9 | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Taurochenodesoxycholic acid 10V, Negative-QTOF | splash10-0002-0000900000-31f17d1d48b7afdf3bae | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Taurochenodesoxycholic acid 20V, Negative-QTOF | splash10-0002-0000900000-a426f175803c68cb8313 | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Taurochenodesoxycholic acid 40V, Negative-QTOF | splash10-000t-7400900000-5a469259d1091a858347 | 2021-09-24 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum |
|
|---|
| Disease References | | Colorectal cancer |
|---|
- Brown DG, Rao S, Weir TL, O'Malia J, Bazan M, Brown RJ, Ryan EP: Metabolomics and metabolic pathway networks from human colorectal cancers, adjacent mucosa, and stool. Cancer Metab. 2016 Jun 6;4:11. doi: 10.1186/s40170-016-0151-y. eCollection 2016. [PubMed:27275383 ]
| | Crohn's disease |
|---|
- Kolho KL, Pessia A, Jaakkola T, de Vos WM, Velagapudi V: Faecal and Serum Metabolomics in Paediatric Inflammatory Bowel Disease. J Crohns Colitis. 2017 Mar 1;11(3):321-334. doi: 10.1093/ecco-jcc/jjw158. [PubMed:27609529 ]
| | Ulcerative colitis |
|---|
- Kolho KL, Pessia A, Jaakkola T, de Vos WM, Velagapudi V: Faecal and Serum Metabolomics in Paediatric Inflammatory Bowel Disease. J Crohns Colitis. 2017 Mar 1;11(3):321-334. doi: 10.1093/ecco-jcc/jjw158. [PubMed:27609529 ]
| | Biliary atresia |
|---|
- Nittono H, Obinata K, Nakatsu N, Watanabe T, Niijima S, Sasaki H, Arisaka O, Kato H, Yabuta K, Miyano T: Sulfated and nonsulfated bile acids in urine of patients with biliary atresia: analysis of bile acids by high-performance liquid chromatography. J Pediatr Gastroenterol Nutr. 1986 Jan;5(1):23-9. [PubMed:3944741 ]
|
|
|---|
| General References | - Bloch CA, Watkins JB: Determination of conjugated bile acids in human bile and duodenal fluid by reverse-phase high-performance liquid chromatography. J Lipid Res. 1978 May;19(4):510-3. [PubMed:659989 ]
- Makino I, Shinozaki K, Nakagawa S, Mashimo K: Measurement of sulfated and nonsulfated bile acids in human serum and urine. J Lipid Res. 1974 Mar;15(2):132-8. [PubMed:4832755 ]
- Pouwels MJ, Tack CJ, Span PN, Olthaar AJ, Sweep CG, Huvers FC, Lutterman JA, Hermus AR: Role of hexosamines in insulin resistance and nutrient sensing in human adipose and muscle tissue. J Clin Endocrinol Metab. 2004 Oct;89(10):5132-7. [PubMed:15472217 ]
- Ijare OB, Somashekar BS, Jadegoud Y, Nagana Gowda GA: 1H and 13C NMR characterization and stereochemical assignments of bile acids in aqueous media. Lipids. 2005 Oct;40(10):1031-41. [PubMed:16382575 ]
- Schwenk M, Hofmann AF, Carlson GL, Carter JA, Coulston F, Greim H: Bile acid conjugation in the chimpanzee: effective sulfation of lithocholic acid. Arch Toxicol. 1978 Apr 27;40(2):109-18. [PubMed:580732 ]
- Bolhuis PA, Sinaasappel M: Bile acid clearance and biocompatibility of XAD-4 haemoperfusion. Int J Artif Organs. 1978 May;1(3):135-41. [PubMed:689756 ]
- Roda A, Pellicciari R, Polimeni C, Cerre C, Forti GC, Sadeghpour B, Sapigni E, Gioacchini AM, Natalini B: Metabolism, pharmacokinetics, and activity of a new 6-fluoro analogue of ursodeoxycholic acid in rats and hamsters. Gastroenterology. 1995 Apr;108(4):1204-14. [PubMed:7698590 ]
- St-Pierre MV, Kullak-Ublick GA, Hagenbuch B, Meier PJ: Transport of bile acids in hepatic and non-hepatic tissues. J Exp Biol. 2001 May;204(Pt 10):1673-86. [PubMed:11316487 ]
- Claudel T, Staels B, Kuipers F: The Farnesoid X receptor: a molecular link between bile acid and lipid and glucose metabolism. Arterioscler Thromb Vasc Biol. 2005 Oct;25(10):2020-30. Epub 2005 Jul 21. [PubMed:16037564 ]
- Chiang JY: Bile acid regulation of hepatic physiology: III. Bile acids and nuclear receptors. Am J Physiol Gastrointest Liver Physiol. 2003 Mar;284(3):G349-56. [PubMed:12576301 ]
- Davis RA, Miyake JH, Hui TY, Spann NJ: Regulation of cholesterol-7alpha-hydroxylase: BAREly missing a SHP. J Lipid Res. 2002 Apr;43(4):533-43. [PubMed:11907135 ]
- Elshenawy S, Pinney SE, Stuart T, Doulias PT, Zura G, Parry S, Elovitz MA, Bennett MJ, Bansal A, Strauss JF 3rd, Ischiropoulos H, Simmons RA: The Metabolomic Signature of the Placenta in Spontaneous Preterm Birth. Int J Mol Sci. 2020 Feb 4;21(3). pii: ijms21031043. doi: 10.3390/ijms21031043. [PubMed:32033212 ]
|
|---|