| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Expected but not Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2021-09-14 15:48:16 UTC |
|---|
| HMDB ID | HMDB0001245 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | dCDP |
|---|
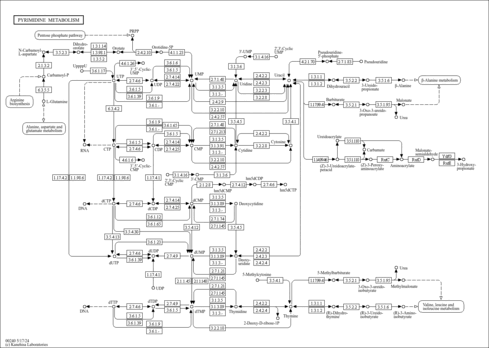
| Description | dCDP, also known as deoxy-CDP, belongs to the class of organic compounds known as organic pyrophosphates. These are organic compounds containing the pyrophosphate oxoanion, with the structure OP([O-])(=O)OP(O)([O-])=O. dCDP is an extremely weak basic (essentially neutral) compound (based on its pKa). dCDP exists in all living species, ranging from bacteria to humans. Within humans, dCDP participates in a number of enzymatic reactions. In particular, dCDP can be biosynthesized from CDP through the action of the enzyme ribonucleoside-diphosphate reductase large subunit. In addition, dCDP can be converted into dCTP; which is mediated by the enzyme nucleoside diphosphate kinase 6. In humans, dCDP is involved in pyrimidine metabolism. Outside of the human body, dCDP has been detected, but not quantified in, several different foods, such as climbing beans, fox grapes, chinese mustards, angelica, and guava. This could make dCDP a potential biomarker for the consumption of these foods. A 2'-deoxycytidine phosphate that is the 2'- deoxy derivative of cytidine 5'-diphosphate (CDP). |
|---|
| Structure | NC1=NC(=O)N(C=C1)[C@H]1C[C@H](O)[C@@H](COP(O)(=O)OP(O)(O)=O)O1 InChI=1S/C9H15N3O10P2/c10-7-1-2-12(9(14)11-7)8-3-5(13)6(21-8)4-20-24(18,19)22-23(15,16)17/h1-2,5-6,8,13H,3-4H2,(H,18,19)(H2,10,11,14)(H2,15,16,17)/t5-,6+,8+/m0/s1 |
|---|
| Synonyms | | Value | Source |
|---|
| 2'-Deoxycytidine 5'-diphosphate | ChEBI | | 2'-Deoxycytidine diphosphate | ChEBI | | D-1beta-Ribofuranosylcytosine diphosphate | ChEBI | | Deoxycytidine diphosphate | ChEBI | | 2'-Deoxycytidine 5'-diphosphoric acid | Generator | | 2'-Deoxycytidine diphosphoric acid | Generator | | D-1b-Ribofuranosylcytosine diphosphate | Generator | | D-1b-Ribofuranosylcytosine diphosphoric acid | Generator | | D-1beta-Ribofuranosylcytosine diphosphoric acid | Generator | | D-1Β-ribofuranosylcytosine diphosphate | Generator | | D-1Β-ribofuranosylcytosine diphosphoric acid | Generator | | Deoxycytidine diphosphoric acid | Generator | | 2'-Deoxy-cytidine 5'-pyrophosphate | HMDB | | 2'-Deoxy-cytidine pyrophosphate | HMDB | | 2'-Deoxycytidine-5'-diphosphate | HMDB | | 4-Amino-1-[2-deoxy-5-O-[hydroxy(phosphonooxy)phosphinyl]-beta-D-erythro-pentofuranosyl]-2(1H)-pyrimidinone | HMDB | | 4-Amino-1-[2-deoxy-5-O-[hydroxy(phosphonooxy)phosphinyl]-beta-delta-erythro-pentofuranosyl]-2(1H)-pyrimidinone | HMDB | | delta-1beta-Ribofuranosylcytosine diphosphate | HMDB | | Deoxy-CDP | HMDB | | Deoxycytidine 5'-diphosphate | HMDB |
|
|---|
| Chemical Formula | C9H15N3O10P2 |
|---|
| Average Molecular Weight | 387.177 |
|---|
| Monoisotopic Molecular Weight | 387.023266739 |
|---|
| IUPAC Name | [({[(2R,3S,5R)-5-(4-amino-2-oxo-1,2-dihydropyrimidin-1-yl)-3-hydroxyoxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy]phosphonic acid |
|---|
| Traditional Name | dCDP |
|---|
| CAS Registry Number | 800-73-7 |
|---|
| SMILES | NC1=NC(=O)N(C=C1)[C@H]1C[C@H](O)[C@@H](COP(O)(=O)OP(O)(O)=O)O1 |
|---|
| InChI Identifier | InChI=1S/C9H15N3O10P2/c10-7-1-2-12(9(14)11-7)8-3-5(13)6(21-8)4-20-24(18,19)22-23(15,16)17/h1-2,5-6,8,13H,3-4H2,(H,18,19)(H2,10,11,14)(H2,15,16,17)/t5-,6+,8+/m0/s1 |
|---|
| InChI Key | FTDHDKPUHBLBTL-SHYZEUOFSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as organic pyrophosphates. These are organic compounds containing the pyrophosphate oxoanion, with the structure OP([O-])(=O)OP(O)([O-])=O. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organic oxoanionic compounds |
|---|
| Sub Class | Organic pyrophosphates |
|---|
| Direct Parent | Organic pyrophosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Organic pyrophosphate
- Aminopyrimidine
- Pyrimidone
- Monoalkyl phosphate
- Hydropyrimidine
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Pyrimidine
- Imidolactam
- Alkyl phosphate
- Heteroaromatic compound
- Tetrahydrofuran
- Secondary alcohol
- Organoheterocyclic compound
- Azacycle
- Oxacycle
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic nitrogen compound
- Amine
- Alcohol
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Not Available | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Experimental Collision Cross Sections |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| dCDP,1TMS,isomer #1 | C[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N)=NC2=O)O[C@@H]1COP(=O)(O)OP(=O)(O)O | 3277.7 | Semi standard non polar | 33892256 | | dCDP,1TMS,isomer #2 | C[Si](C)(C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(N)=NC2=O)C[C@@H]1O)OP(=O)(O)O | 3323.2 | Semi standard non polar | 33892256 | | dCDP,1TMS,isomer #3 | C[Si](C)(C)OP(=O)(O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(N)=NC2=O)C[C@@H]1O | 3311.8 | Semi standard non polar | 33892256 | | dCDP,1TMS,isomer #4 | C[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O)O)O2)C=C1 | 3340.8 | Semi standard non polar | 33892256 | | dCDP,2TMS,isomer #1 | C[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N)=NC2=O)O[C@@H]1COP(=O)(O[Si](C)(C)C)OP(=O)(O)O | 3248.8 | Semi standard non polar | 33892256 | | dCDP,2TMS,isomer #1 | C[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N)=NC2=O)O[C@@H]1COP(=O)(O[Si](C)(C)C)OP(=O)(O)O | 3141.5 | Standard non polar | 33892256 | | dCDP,2TMS,isomer #1 | C[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N)=NC2=O)O[C@@H]1COP(=O)(O[Si](C)(C)C)OP(=O)(O)O | 5192.9 | Standard polar | 33892256 | | dCDP,2TMS,isomer #2 | C[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N)=NC2=O)O[C@@H]1COP(=O)(O)OP(=O)(O)O[Si](C)(C)C | 3242.2 | Semi standard non polar | 33892256 | | dCDP,2TMS,isomer #2 | C[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N)=NC2=O)O[C@@H]1COP(=O)(O)OP(=O)(O)O[Si](C)(C)C | 3132.6 | Standard non polar | 33892256 | | dCDP,2TMS,isomer #2 | C[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N)=NC2=O)O[C@@H]1COP(=O)(O)OP(=O)(O)O[Si](C)(C)C | 5190.1 | Standard polar | 33892256 | | dCDP,2TMS,isomer #3 | C[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O[Si](C)(C)C)[C@@H](COP(=O)(O)OP(=O)(O)O)O2)C=C1 | 3281.6 | Semi standard non polar | 33892256 | | dCDP,2TMS,isomer #3 | C[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O[Si](C)(C)C)[C@@H](COP(=O)(O)OP(=O)(O)O)O2)C=C1 | 3181.5 | Standard non polar | 33892256 | | dCDP,2TMS,isomer #3 | C[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O[Si](C)(C)C)[C@@H](COP(=O)(O)OP(=O)(O)O)O2)C=C1 | 5270.3 | Standard polar | 33892256 | | dCDP,2TMS,isomer #4 | C[Si](C)(C)OP(=O)(O)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(N)=NC2=O)C[C@@H]1O)O[Si](C)(C)C | 3265.5 | Semi standard non polar | 33892256 | | dCDP,2TMS,isomer #4 | C[Si](C)(C)OP(=O)(O)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(N)=NC2=O)C[C@@H]1O)O[Si](C)(C)C | 3190.5 | Standard non polar | 33892256 | | dCDP,2TMS,isomer #4 | C[Si](C)(C)OP(=O)(O)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(N)=NC2=O)C[C@@H]1O)O[Si](C)(C)C | 4933.7 | Standard polar | 33892256 | | dCDP,2TMS,isomer #5 | C[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)O)O2)C=C1 | 3343.0 | Semi standard non polar | 33892256 | | dCDP,2TMS,isomer #5 | C[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)O)O2)C=C1 | 3234.8 | Standard non polar | 33892256 | | dCDP,2TMS,isomer #5 | C[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)O)O2)C=C1 | 4969.7 | Standard polar | 33892256 | | dCDP,2TMS,isomer #6 | C[Si](C)(C)OP(=O)(O[Si](C)(C)C)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(N)=NC2=O)C[C@@H]1O | 3275.5 | Semi standard non polar | 33892256 | | dCDP,2TMS,isomer #6 | C[Si](C)(C)OP(=O)(O[Si](C)(C)C)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(N)=NC2=O)C[C@@H]1O | 3184.4 | Standard non polar | 33892256 | | dCDP,2TMS,isomer #6 | C[Si](C)(C)OP(=O)(O[Si](C)(C)C)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(N)=NC2=O)C[C@@H]1O | 5014.0 | Standard polar | 33892256 | | dCDP,2TMS,isomer #7 | C[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O)O[Si](C)(C)C)O2)C=C1 | 3327.2 | Semi standard non polar | 33892256 | | dCDP,2TMS,isomer #7 | C[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O)O[Si](C)(C)C)O2)C=C1 | 3228.4 | Standard non polar | 33892256 | | dCDP,2TMS,isomer #7 | C[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O)O[Si](C)(C)C)O2)C=C1 | 5003.9 | Standard polar | 33892256 | | dCDP,2TMS,isomer #8 | C[Si](C)(C)N(C1=NC(=O)N([C@H]2C[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O)O)O2)C=C1)[Si](C)(C)C | 3320.9 | Semi standard non polar | 33892256 | | dCDP,2TMS,isomer #8 | C[Si](C)(C)N(C1=NC(=O)N([C@H]2C[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O)O)O2)C=C1)[Si](C)(C)C | 3252.7 | Standard non polar | 33892256 | | dCDP,2TMS,isomer #8 | C[Si](C)(C)N(C1=NC(=O)N([C@H]2C[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O)O)O2)C=C1)[Si](C)(C)C | 5208.9 | Standard polar | 33892256 | | dCDP,3TMS,isomer #1 | C[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N)=NC2=O)O[C@@H]1COP(=O)(O[Si](C)(C)C)OP(=O)(O)O[Si](C)(C)C | 3182.9 | Semi standard non polar | 33892256 | | dCDP,3TMS,isomer #1 | C[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N)=NC2=O)O[C@@H]1COP(=O)(O[Si](C)(C)C)OP(=O)(O)O[Si](C)(C)C | 3162.3 | Standard non polar | 33892256 | | dCDP,3TMS,isomer #1 | C[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N)=NC2=O)O[C@@H]1COP(=O)(O[Si](C)(C)C)OP(=O)(O)O[Si](C)(C)C | 4782.7 | Standard polar | 33892256 | | dCDP,3TMS,isomer #10 | C[Si](C)(C)OP(=O)(O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O)C[C@@H]1O | 3266.0 | Semi standard non polar | 33892256 | | dCDP,3TMS,isomer #10 | C[Si](C)(C)OP(=O)(O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O)C[C@@H]1O | 3306.1 | Standard non polar | 33892256 | | dCDP,3TMS,isomer #10 | C[Si](C)(C)OP(=O)(O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O)C[C@@H]1O | 4672.6 | Standard polar | 33892256 | | dCDP,3TMS,isomer #2 | C[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O[Si](C)(C)C)[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)O)O2)C=C1 | 3260.7 | Semi standard non polar | 33892256 | | dCDP,3TMS,isomer #2 | C[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O[Si](C)(C)C)[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)O)O2)C=C1 | 3240.6 | Standard non polar | 33892256 | | dCDP,3TMS,isomer #2 | C[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O[Si](C)(C)C)[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)O)O2)C=C1 | 4717.7 | Standard polar | 33892256 | | dCDP,3TMS,isomer #3 | C[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N)=NC2=O)O[C@@H]1COP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 3193.5 | Semi standard non polar | 33892256 | | dCDP,3TMS,isomer #3 | C[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N)=NC2=O)O[C@@H]1COP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 3156.5 | Standard non polar | 33892256 | | dCDP,3TMS,isomer #3 | C[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N)=NC2=O)O[C@@H]1COP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 4831.0 | Standard polar | 33892256 | | dCDP,3TMS,isomer #4 | C[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O[Si](C)(C)C)[C@@H](COP(=O)(O)OP(=O)(O)O[Si](C)(C)C)O2)C=C1 | 3260.3 | Semi standard non polar | 33892256 | | dCDP,3TMS,isomer #4 | C[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O[Si](C)(C)C)[C@@H](COP(=O)(O)OP(=O)(O)O[Si](C)(C)C)O2)C=C1 | 3228.5 | Standard non polar | 33892256 | | dCDP,3TMS,isomer #4 | C[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O[Si](C)(C)C)[C@@H](COP(=O)(O)OP(=O)(O)O[Si](C)(C)C)O2)C=C1 | 4770.4 | Standard polar | 33892256 | | dCDP,3TMS,isomer #5 | C[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O)O[C@@H]1COP(=O)(O)OP(=O)(O)O | 3214.1 | Semi standard non polar | 33892256 | | dCDP,3TMS,isomer #5 | C[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O)O[C@@H]1COP(=O)(O)OP(=O)(O)O | 3250.2 | Standard non polar | 33892256 | | dCDP,3TMS,isomer #5 | C[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O)O[C@@H]1COP(=O)(O)OP(=O)(O)O | 4901.7 | Standard polar | 33892256 | | dCDP,3TMS,isomer #6 | C[Si](C)(C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(N)=NC2=O)C[C@@H]1O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 3230.2 | Semi standard non polar | 33892256 | | dCDP,3TMS,isomer #6 | C[Si](C)(C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(N)=NC2=O)C[C@@H]1O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 3222.5 | Standard non polar | 33892256 | | dCDP,3TMS,isomer #6 | C[Si](C)(C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(N)=NC2=O)C[C@@H]1O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 4566.9 | Standard polar | 33892256 | | dCDP,3TMS,isomer #7 | C[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)O[Si](C)(C)C)O2)C=C1 | 3314.4 | Semi standard non polar | 33892256 | | dCDP,3TMS,isomer #7 | C[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)O[Si](C)(C)C)O2)C=C1 | 3292.7 | Standard non polar | 33892256 | | dCDP,3TMS,isomer #7 | C[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)O[Si](C)(C)C)O2)C=C1 | 4500.1 | Standard polar | 33892256 | | dCDP,3TMS,isomer #8 | C[Si](C)(C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O)C[C@@H]1O)OP(=O)(O)O | 3274.5 | Semi standard non polar | 33892256 | | dCDP,3TMS,isomer #8 | C[Si](C)(C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O)C[C@@H]1O)OP(=O)(O)O | 3319.2 | Standard non polar | 33892256 | | dCDP,3TMS,isomer #8 | C[Si](C)(C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O)C[C@@H]1O)OP(=O)(O)O | 4614.0 | Standard polar | 33892256 | | dCDP,3TMS,isomer #9 | C[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O2)C=C1 | 3314.2 | Semi standard non polar | 33892256 | | dCDP,3TMS,isomer #9 | C[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O2)C=C1 | 3283.3 | Standard non polar | 33892256 | | dCDP,3TMS,isomer #9 | C[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O2)C=C1 | 4518.4 | Standard polar | 33892256 | | dCDP,4TMS,isomer #1 | C[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N)=NC2=O)O[C@@H]1COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 3172.4 | Semi standard non polar | 33892256 | | dCDP,4TMS,isomer #1 | C[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N)=NC2=O)O[C@@H]1COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 3155.5 | Standard non polar | 33892256 | | dCDP,4TMS,isomer #1 | C[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N)=NC2=O)O[C@@H]1COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 4457.0 | Standard polar | 33892256 | | dCDP,4TMS,isomer #2 | C[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O[Si](C)(C)C)[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)O[Si](C)(C)C)O2)C=C1 | 3274.3 | Semi standard non polar | 33892256 | | dCDP,4TMS,isomer #2 | C[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O[Si](C)(C)C)[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)O[Si](C)(C)C)O2)C=C1 | 3251.7 | Standard non polar | 33892256 | | dCDP,4TMS,isomer #2 | C[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O[Si](C)(C)C)[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)O[Si](C)(C)C)O2)C=C1 | 4311.8 | Standard polar | 33892256 | | dCDP,4TMS,isomer #3 | C[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O)O[C@@H]1COP(=O)(O[Si](C)(C)C)OP(=O)(O)O | 3213.0 | Semi standard non polar | 33892256 | | dCDP,4TMS,isomer #3 | C[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O)O[C@@H]1COP(=O)(O[Si](C)(C)C)OP(=O)(O)O | 3272.4 | Standard non polar | 33892256 | | dCDP,4TMS,isomer #3 | C[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O)O[C@@H]1COP(=O)(O[Si](C)(C)C)OP(=O)(O)O | 4373.3 | Standard polar | 33892256 | | dCDP,4TMS,isomer #4 | C[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O[Si](C)(C)C)[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O2)C=C1 | 3271.3 | Semi standard non polar | 33892256 | | dCDP,4TMS,isomer #4 | C[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O[Si](C)(C)C)[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O2)C=C1 | 3246.9 | Standard non polar | 33892256 | | dCDP,4TMS,isomer #4 | C[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O[Si](C)(C)C)[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O2)C=C1 | 4315.0 | Standard polar | 33892256 | | dCDP,4TMS,isomer #5 | C[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O)O[C@@H]1COP(=O)(O)OP(=O)(O)O[Si](C)(C)C | 3209.5 | Semi standard non polar | 33892256 | | dCDP,4TMS,isomer #5 | C[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O)O[C@@H]1COP(=O)(O)OP(=O)(O)O[Si](C)(C)C | 3257.5 | Standard non polar | 33892256 | | dCDP,4TMS,isomer #5 | C[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O)O[C@@H]1COP(=O)(O)OP(=O)(O)O[Si](C)(C)C | 4441.0 | Standard polar | 33892256 | | dCDP,4TMS,isomer #6 | C[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O2)C=C1 | 3319.6 | Semi standard non polar | 33892256 | | dCDP,4TMS,isomer #6 | C[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O2)C=C1 | 3287.8 | Standard non polar | 33892256 | | dCDP,4TMS,isomer #6 | C[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O2)C=C1 | 4070.1 | Standard polar | 33892256 | | dCDP,4TMS,isomer #7 | C[Si](C)(C)OP(=O)(O)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O)C[C@@H]1O)O[Si](C)(C)C | 3269.3 | Semi standard non polar | 33892256 | | dCDP,4TMS,isomer #7 | C[Si](C)(C)OP(=O)(O)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O)C[C@@H]1O)O[Si](C)(C)C | 3316.1 | Standard non polar | 33892256 | | dCDP,4TMS,isomer #7 | C[Si](C)(C)OP(=O)(O)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O)C[C@@H]1O)O[Si](C)(C)C | 4200.6 | Standard polar | 33892256 | | dCDP,4TMS,isomer #8 | C[Si](C)(C)OP(=O)(O[Si](C)(C)C)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O)C[C@@H]1O | 3277.5 | Semi standard non polar | 33892256 | | dCDP,4TMS,isomer #8 | C[Si](C)(C)OP(=O)(O[Si](C)(C)C)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O)C[C@@H]1O | 3310.5 | Standard non polar | 33892256 | | dCDP,4TMS,isomer #8 | C[Si](C)(C)OP(=O)(O[Si](C)(C)C)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O)C[C@@H]1O | 4197.1 | Standard polar | 33892256 | | dCDP,5TMS,isomer #1 | C[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O[Si](C)(C)C)[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O2)C=C1 | 3275.2 | Semi standard non polar | 33892256 | | dCDP,5TMS,isomer #1 | C[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O[Si](C)(C)C)[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O2)C=C1 | 3227.0 | Standard non polar | 33892256 | | dCDP,5TMS,isomer #1 | C[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O[Si](C)(C)C)[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O2)C=C1 | 3884.5 | Standard polar | 33892256 | | dCDP,5TMS,isomer #2 | C[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O)O[C@@H]1COP(=O)(O[Si](C)(C)C)OP(=O)(O)O[Si](C)(C)C | 3236.8 | Semi standard non polar | 33892256 | | dCDP,5TMS,isomer #2 | C[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O)O[C@@H]1COP(=O)(O[Si](C)(C)C)OP(=O)(O)O[Si](C)(C)C | 3237.8 | Standard non polar | 33892256 | | dCDP,5TMS,isomer #2 | C[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O)O[C@@H]1COP(=O)(O[Si](C)(C)C)OP(=O)(O)O[Si](C)(C)C | 4000.5 | Standard polar | 33892256 | | dCDP,5TMS,isomer #3 | C[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O)O[C@@H]1COP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 3235.5 | Semi standard non polar | 33892256 | | dCDP,5TMS,isomer #3 | C[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O)O[C@@H]1COP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 3229.7 | Standard non polar | 33892256 | | dCDP,5TMS,isomer #3 | C[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O)O[C@@H]1COP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 3987.6 | Standard polar | 33892256 | | dCDP,5TMS,isomer #4 | C[Si](C)(C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O)C[C@@H]1O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 3285.6 | Semi standard non polar | 33892256 | | dCDP,5TMS,isomer #4 | C[Si](C)(C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O)C[C@@H]1O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 3270.4 | Standard non polar | 33892256 | | dCDP,5TMS,isomer #4 | C[Si](C)(C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O)C[C@@H]1O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 3785.8 | Standard polar | 33892256 | | dCDP,6TMS,isomer #1 | C[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O)O[C@@H]1COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 3291.4 | Semi standard non polar | 33892256 | | dCDP,6TMS,isomer #1 | C[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O)O[C@@H]1COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 3188.7 | Standard non polar | 33892256 | | dCDP,6TMS,isomer #1 | C[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N([Si](C)(C)C)[Si](C)(C)C)=NC2=O)O[C@@H]1COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 3629.7 | Standard polar | 33892256 | | dCDP,1TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N)=NC2=O)O[C@@H]1COP(=O)(O)OP(=O)(O)O | 3528.8 | Semi standard non polar | 33892256 | | dCDP,1TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(N)=NC2=O)C[C@@H]1O)OP(=O)(O)O | 3564.6 | Semi standard non polar | 33892256 | | dCDP,1TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OP(=O)(O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(N)=NC2=O)C[C@@H]1O | 3552.8 | Semi standard non polar | 33892256 | | dCDP,1TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O)O)O2)C=C1 | 3610.6 | Semi standard non polar | 33892256 | | dCDP,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N)=NC2=O)O[C@@H]1COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O | 3700.8 | Semi standard non polar | 33892256 | | dCDP,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N)=NC2=O)O[C@@H]1COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O | 3533.6 | Standard non polar | 33892256 | | dCDP,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N)=NC2=O)O[C@@H]1COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O | 5246.5 | Standard polar | 33892256 | | dCDP,2TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N)=NC2=O)O[C@@H]1COP(=O)(O)OP(=O)(O)O[Si](C)(C)C(C)(C)C | 3693.0 | Semi standard non polar | 33892256 | | dCDP,2TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N)=NC2=O)O[C@@H]1COP(=O)(O)OP(=O)(O)O[Si](C)(C)C(C)(C)C | 3533.1 | Standard non polar | 33892256 | | dCDP,2TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N)=NC2=O)O[C@@H]1COP(=O)(O)OP(=O)(O)O[Si](C)(C)C(C)(C)C | 5246.3 | Standard polar | 33892256 | | dCDP,2TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](COP(=O)(O)OP(=O)(O)O)O2)C=C1 | 3772.6 | Semi standard non polar | 33892256 | | dCDP,2TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](COP(=O)(O)OP(=O)(O)O)O2)C=C1 | 3599.9 | Standard non polar | 33892256 | | dCDP,2TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](COP(=O)(O)OP(=O)(O)O)O2)C=C1 | 5230.1 | Standard polar | 33892256 | | dCDP,2TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)OP(=O)(O)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(N)=NC2=O)C[C@@H]1O)O[Si](C)(C)C(C)(C)C | 3705.1 | Semi standard non polar | 33892256 | | dCDP,2TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)OP(=O)(O)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(N)=NC2=O)C[C@@H]1O)O[Si](C)(C)C(C)(C)C | 3565.6 | Standard non polar | 33892256 | | dCDP,2TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)OP(=O)(O)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(N)=NC2=O)C[C@@H]1O)O[Si](C)(C)C(C)(C)C | 5040.4 | Standard polar | 33892256 | | dCDP,2TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O)O2)C=C1 | 3807.4 | Semi standard non polar | 33892256 | | dCDP,2TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O)O2)C=C1 | 3622.0 | Standard non polar | 33892256 | | dCDP,2TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O)O2)C=C1 | 5031.1 | Standard polar | 33892256 | | dCDP,2TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)OP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(N)=NC2=O)C[C@@H]1O | 3716.5 | Semi standard non polar | 33892256 | | dCDP,2TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)OP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(N)=NC2=O)C[C@@H]1O | 3539.5 | Standard non polar | 33892256 | | dCDP,2TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)OP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(N)=NC2=O)C[C@@H]1O | 5109.0 | Standard polar | 33892256 | | dCDP,2TBDMS,isomer #7 | CC(C)(C)[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O)O[Si](C)(C)C(C)(C)C)O2)C=C1 | 3808.6 | Semi standard non polar | 33892256 | | dCDP,2TBDMS,isomer #7 | CC(C)(C)[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O)O[Si](C)(C)C(C)(C)C)O2)C=C1 | 3626.1 | Standard non polar | 33892256 | | dCDP,2TBDMS,isomer #7 | CC(C)(C)[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O)O[Si](C)(C)C(C)(C)C)O2)C=C1 | 5068.9 | Standard polar | 33892256 | | dCDP,2TBDMS,isomer #8 | CC(C)(C)[Si](C)(C)N(C1=NC(=O)N([C@H]2C[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O)O)O2)C=C1)[Si](C)(C)C(C)(C)C | 3728.0 | Semi standard non polar | 33892256 | | dCDP,2TBDMS,isomer #8 | CC(C)(C)[Si](C)(C)N(C1=NC(=O)N([C@H]2C[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O)O)O2)C=C1)[Si](C)(C)C(C)(C)C | 3632.3 | Standard non polar | 33892256 | | dCDP,2TBDMS,isomer #8 | CC(C)(C)[Si](C)(C)N(C1=NC(=O)N([C@H]2C[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O)O)O2)C=C1)[Si](C)(C)C(C)(C)C | 5150.8 | Standard polar | 33892256 | | dCDP,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N)=NC2=O)O[C@@H]1COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O[Si](C)(C)C(C)(C)C | 3858.4 | Semi standard non polar | 33892256 | | dCDP,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N)=NC2=O)O[C@@H]1COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O[Si](C)(C)C(C)(C)C | 3680.5 | Standard non polar | 33892256 | | dCDP,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N)=NC2=O)O[C@@H]1COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O[Si](C)(C)C(C)(C)C | 4887.0 | Standard polar | 33892256 | | dCDP,3TBDMS,isomer #10 | CC(C)(C)[Si](C)(C)OP(=O)(O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)=NC2=O)C[C@@H]1O | 3895.7 | Semi standard non polar | 33892256 | | dCDP,3TBDMS,isomer #10 | CC(C)(C)[Si](C)(C)OP(=O)(O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)=NC2=O)C[C@@H]1O | 3851.4 | Standard non polar | 33892256 | | dCDP,3TBDMS,isomer #10 | CC(C)(C)[Si](C)(C)OP(=O)(O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)=NC2=O)C[C@@H]1O | 4792.9 | Standard polar | 33892256 | | dCDP,3TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O)O2)C=C1 | 3938.2 | Semi standard non polar | 33892256 | | dCDP,3TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O)O2)C=C1 | 3789.1 | Standard non polar | 33892256 | | dCDP,3TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O)O2)C=C1 | 4847.0 | Standard polar | 33892256 | | dCDP,3TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N)=NC2=O)O[C@@H]1COP(=O)(O)OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 3866.4 | Semi standard non polar | 33892256 | | dCDP,3TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N)=NC2=O)O[C@@H]1COP(=O)(O)OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 3656.0 | Standard non polar | 33892256 | | dCDP,3TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N)=NC2=O)O[C@@H]1COP(=O)(O)OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 4925.8 | Standard polar | 33892256 | | dCDP,3TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](COP(=O)(O)OP(=O)(O)O[Si](C)(C)C(C)(C)C)O2)C=C1 | 3938.4 | Semi standard non polar | 33892256 | | dCDP,3TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](COP(=O)(O)OP(=O)(O)O[Si](C)(C)C(C)(C)C)O2)C=C1 | 3794.4 | Standard non polar | 33892256 | | dCDP,3TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](COP(=O)(O)OP(=O)(O)O[Si](C)(C)C(C)(C)C)O2)C=C1 | 4894.0 | Standard polar | 33892256 | | dCDP,3TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)=NC2=O)O[C@@H]1COP(=O)(O)OP(=O)(O)O | 3847.6 | Semi standard non polar | 33892256 | | dCDP,3TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)=NC2=O)O[C@@H]1COP(=O)(O)OP(=O)(O)O | 3811.9 | Standard non polar | 33892256 | | dCDP,3TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)=NC2=O)O[C@@H]1COP(=O)(O)OP(=O)(O)O | 4948.9 | Standard polar | 33892256 | | dCDP,3TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(N)=NC2=O)C[C@@H]1O)OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 3862.2 | Semi standard non polar | 33892256 | | dCDP,3TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(N)=NC2=O)C[C@@H]1O)OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 3686.4 | Standard non polar | 33892256 | | dCDP,3TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(N)=NC2=O)C[C@@H]1O)OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 4690.5 | Standard polar | 33892256 | | dCDP,3TBDMS,isomer #7 | CC(C)(C)[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O[Si](C)(C)C(C)(C)C)O2)C=C1 | 3970.0 | Semi standard non polar | 33892256 | | dCDP,3TBDMS,isomer #7 | CC(C)(C)[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O[Si](C)(C)C(C)(C)C)O2)C=C1 | 3819.7 | Standard non polar | 33892256 | | dCDP,3TBDMS,isomer #7 | CC(C)(C)[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O[Si](C)(C)C(C)(C)C)O2)C=C1 | 4670.8 | Standard polar | 33892256 | | dCDP,3TBDMS,isomer #8 | CC(C)(C)[Si](C)(C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)=NC2=O)C[C@@H]1O)OP(=O)(O)O | 3902.4 | Semi standard non polar | 33892256 | | dCDP,3TBDMS,isomer #8 | CC(C)(C)[Si](C)(C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)=NC2=O)C[C@@H]1O)OP(=O)(O)O | 3847.4 | Standard non polar | 33892256 | | dCDP,3TBDMS,isomer #8 | CC(C)(C)[Si](C)(C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)=NC2=O)C[C@@H]1O)OP(=O)(O)O | 4746.7 | Standard polar | 33892256 | | dCDP,3TBDMS,isomer #9 | CC(C)(C)[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)O2)C=C1 | 3973.2 | Semi standard non polar | 33892256 | | dCDP,3TBDMS,isomer #9 | CC(C)(C)[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)O2)C=C1 | 3787.0 | Standard non polar | 33892256 | | dCDP,3TBDMS,isomer #9 | CC(C)(C)[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)O2)C=C1 | 4692.7 | Standard polar | 33892256 | | dCDP,4TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N)=NC2=O)O[C@@H]1COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 3988.9 | Semi standard non polar | 33892256 | | dCDP,4TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N)=NC2=O)O[C@@H]1COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 3716.9 | Standard non polar | 33892256 | | dCDP,4TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N)=NC2=O)O[C@@H]1COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 4568.7 | Standard polar | 33892256 | | dCDP,4TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O[Si](C)(C)C(C)(C)C)O2)C=C1 | 4100.2 | Semi standard non polar | 33892256 | | dCDP,4TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O[Si](C)(C)C(C)(C)C)O2)C=C1 | 3894.6 | Standard non polar | 33892256 | | dCDP,4TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O[Si](C)(C)C(C)(C)C)O2)C=C1 | 4478.7 | Standard polar | 33892256 | | dCDP,4TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)=NC2=O)O[C@@H]1COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O | 4042.1 | Semi standard non polar | 33892256 | | dCDP,4TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)=NC2=O)O[C@@H]1COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O | 3935.8 | Standard non polar | 33892256 | | dCDP,4TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)=NC2=O)O[C@@H]1COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O | 4506.7 | Standard polar | 33892256 | | dCDP,4TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)O2)C=C1 | 4101.5 | Semi standard non polar | 33892256 | | dCDP,4TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)O2)C=C1 | 3870.1 | Standard non polar | 33892256 | | dCDP,4TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)O2)C=C1 | 4490.4 | Standard polar | 33892256 | | dCDP,4TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)=NC2=O)O[C@@H]1COP(=O)(O)OP(=O)(O)O[Si](C)(C)C(C)(C)C | 4036.2 | Semi standard non polar | 33892256 | | dCDP,4TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)=NC2=O)O[C@@H]1COP(=O)(O)OP(=O)(O)O[Si](C)(C)C(C)(C)C | 3932.9 | Standard non polar | 33892256 | | dCDP,4TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)O[C@H]1C[C@H](N2C=CC(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)=NC2=O)O[C@@H]1COP(=O)(O)OP(=O)(O)O[Si](C)(C)C(C)(C)C | 4575.3 | Standard polar | 33892256 | | dCDP,4TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)O2)C=C1 | 4130.7 | Semi standard non polar | 33892256 | | dCDP,4TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)O2)C=C1 | 3885.6 | Standard non polar | 33892256 | | dCDP,4TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)NC1=NC(=O)N([C@H]2C[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)O2)C=C1 | 4286.3 | Standard polar | 33892256 | | dCDP,4TBDMS,isomer #7 | CC(C)(C)[Si](C)(C)OP(=O)(O)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)=NC2=O)C[C@@H]1O)O[Si](C)(C)C(C)(C)C | 4061.6 | Semi standard non polar | 33892256 | | dCDP,4TBDMS,isomer #7 | CC(C)(C)[Si](C)(C)OP(=O)(O)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)=NC2=O)C[C@@H]1O)O[Si](C)(C)C(C)(C)C | 3970.1 | Standard non polar | 33892256 | | dCDP,4TBDMS,isomer #7 | CC(C)(C)[Si](C)(C)OP(=O)(O)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)=NC2=O)C[C@@H]1O)O[Si](C)(C)C(C)(C)C | 4361.9 | Standard polar | 33892256 | | dCDP,4TBDMS,isomer #8 | CC(C)(C)[Si](C)(C)OP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)=NC2=O)C[C@@H]1O | 4066.5 | Semi standard non polar | 33892256 | | dCDP,4TBDMS,isomer #8 | CC(C)(C)[Si](C)(C)OP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)=NC2=O)C[C@@H]1O | 3945.2 | Standard non polar | 33892256 | | dCDP,4TBDMS,isomer #8 | CC(C)(C)[Si](C)(C)OP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)=NC2=O)C[C@@H]1O | 4366.9 | Standard polar | 33892256 |
|
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - dCDP GC-MS (Non-derivatized) - 70eV, Positive | splash10-002e-7912000000-ef0991eaee32b505e016 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - dCDP GC-MS (1 TMS) - 70eV, Positive | splash10-0006-9851100000-62426a0730b483cea09b | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - dCDP GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - dCDP GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental LC-MS/MS | LC-MS/MS Spectrum - dCDP LC-ESI-QTOF , negative-QTOF | splash10-0570-9418000000-2d9af3073a3dc2deba5d | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - dCDP , negative-QTOF | splash10-056r-9511000000-84defb0c7de79c169ebf | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - dCDP LC-ESI-QTOF , positive-QTOF | splash10-000i-0409000000-e5af2ab8466aa7c560d3 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - dCDP LC-ESI-QTOF , positive-QTOF | splash10-0a4i-0903000000-1de5fa4ac24e19830f39 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - dCDP 10V, Negative-QTOF | splash10-000i-0009000000-4aa3ce1bf37251d7fc83 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - dCDP 20V, Negative-QTOF | splash10-056r-9435000000-55592321586c623c4830 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - dCDP 30V, Positive-QTOF | splash10-0a4i-0903000000-1de5fa4ac24e19830f39 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - dCDP 35V, Negative-QTOF | splash10-056r-9411000000-84bbde6c497548c746d1 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - dCDP 40V, Negative-QTOF | splash10-004i-9100000000-63f9c6b289fb024c58fa | 2021-09-20 | HMDB team, MONA | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - dCDP 10V, Positive-QTOF | splash10-03di-0900000000-e584f4f2cb1a8a01a7e7 | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - dCDP 20V, Positive-QTOF | splash10-03di-3910000000-d34603d21a6d1d52ae1f | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - dCDP 40V, Positive-QTOF | splash10-03di-5900000000-52ac41f181e1d5fbf96a | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - dCDP 10V, Negative-QTOF | splash10-000f-4209000000-ea241dab90201ce7963e | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - dCDP 20V, Negative-QTOF | splash10-004i-9601000000-55120f468bf1ebba66cd | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - dCDP 40V, Negative-QTOF | splash10-004i-9100000000-bd81788fb13a599a4f60 | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - dCDP 10V, Positive-QTOF | splash10-03di-0900000000-63028e00ccb9887f46f5 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - dCDP 20V, Positive-QTOF | splash10-03di-1900000000-9b943638c706a10be8db | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - dCDP 40V, Positive-QTOF | splash10-03di-3910000000-f5ebfcca0e4ec5d76afa | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - dCDP 10V, Negative-QTOF | splash10-000i-0409000000-47d71d5707b30d7b3ad5 | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - dCDP 20V, Negative-QTOF | splash10-004i-9324000000-25bfbc45e3248b83b219 | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - dCDP 40V, Negative-QTOF | splash10-004i-9000000000-a5e502a2627af2048a1f | 2021-09-25 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum |
|
|---|