| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected but not Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2021-09-14 15:38:57 UTC |
|---|
| HMDB ID | HMDB0001369 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Pyrroline hydroxycarboxylic acid |
|---|
| Description | Pyrroline hydroxycarboxylic acid is a metabolite identified in the urine of patients with type II hyperprolinemia. (OMIM 239510 ). The urinary excretion of Pyrroline hydroxycarboxylic acid increased in hyperprolinemic patients but not in healthy controls during oral loading of hydroxyproline and hydroxyproline-ornithine. (PMID: 533224 ). Hyperprolinemia type II (HP II) is a rare inherited metabolic disease due to the deficiency of pyroline-5-carboxylate dehydrogenase. It is generally believed to be a benign condition although some patients have neurological problems such as refractory convulsions. (PMID: 15214748 ). The oxidation of pyrroline-carboxylate generates glutamate and pyrroline-hydroxycarboxylate, a reaction catalyzed by hydroxyproline oxidase (PMID: 500817 ). |
|---|
| Structure | InChI=1S/C5H7NO3/c7-3-1-4(5(8)9)6-2-3/h2-4,7H,1H2,(H,8,9) |
|---|
| Synonyms | | Value | Source |
|---|
| Pyrroline hydroxycarboxylate | Generator | | 3-Hydroxy-L-1-pyrroline-5-carboxylate | HMDB | | L-1-Pyrroline 3-hydroxy-5-carboxylate | HMDB | | L-1-Pyrroline-3-hydroxy-5-carboxylate | HMDB | | L-Delta1-Pyrroline 3-hydroxy-5-carboxylate | HMDB | | Pyrroline-hydroxy-carboxylate | HMDB | | 3,4-Dihydro-3-hydroxy-2H-pyrrole-5-carboxylic acid | HMDB | | 3-Hydroxy delta 1-pyrroline-5-carboxylic acid | HMDB | | 3-Hydroxy delta 1-pyrroline-5-carboxylic acid, anion | HMDB |
|
|---|
| Chemical Formula | C5H7NO3 |
|---|
| Average Molecular Weight | 129.114 |
|---|
| Monoisotopic Molecular Weight | 129.042593095 |
|---|
| IUPAC Name | 4-hydroxy-3,4-dihydro-2H-pyrrole-2-carboxylic acid |
|---|
| Traditional Name | pyrroline-hydroxy-carboxylate |
|---|
| CAS Registry Number | 22573-88-2 |
|---|
| SMILES | OC1CC(N=C1)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C5H7NO3/c7-3-1-4(5(8)9)6-2-3/h2-4,7H,1H2,(H,8,9) |
|---|
| InChI Key | WFOFKRKDDKGRIK-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as alpha amino acids and derivatives. These are amino acids in which the amino group is attached to the carbon atom immediately adjacent to the carboxylate group (alpha carbon), or a derivative thereof. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Alpha amino acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-amino acid or derivatives
- Pyrroline carboxylic acid
- Pyrroline carboxylic acid or derivatives
- Pyrroline
- Secondary alcohol
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Propargyl-type 1,3-dipolar organic compound
- Organic 1,3-dipolar compound
- Organoheterocyclic compound
- Azacycle
- Organopnictogen compound
- Organic oxygen compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxide
- Hydrocarbon derivative
- Organic nitrogen compound
- Carbonyl group
- Alcohol
- Imine
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Not Available |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| Pyrroline hydroxycarboxylic acid,1TMS,isomer #1 | C[Si](C)(C)OC1C=NC(C(=O)O)C1 | 1393.9 | Semi standard non polar | 33892256 | | Pyrroline hydroxycarboxylic acid,1TMS,isomer #2 | C[Si](C)(C)OC(=O)C1CC(O)C=N1 | 1277.1 | Semi standard non polar | 33892256 | | Pyrroline hydroxycarboxylic acid,2TMS,isomer #1 | C[Si](C)(C)OC(=O)C1CC(O[Si](C)(C)C)C=N1 | 1407.7 | Semi standard non polar | 33892256 | | Pyrroline hydroxycarboxylic acid,1TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC1C=NC(C(=O)O)C1 | 1640.6 | Semi standard non polar | 33892256 | | Pyrroline hydroxycarboxylic acid,1TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OC(=O)C1CC(O)C=N1 | 1549.4 | Semi standard non polar | 33892256 | | Pyrroline hydroxycarboxylic acid,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)C1CC(O[Si](C)(C)C(C)(C)C)C=N1 | 1839.4 | Semi standard non polar | 33892256 |
|
|---|
| Spectra |
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - Pyrroline hydroxycarboxylic acid GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4l-9100000000-81454e4422185c7858e4 | 2016-09-22 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Pyrroline hydroxycarboxylic acid GC-MS (2 TMS) - 70eV, Positive | splash10-0a4i-5910000000-0d5241ee660e7a60b0f9 | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Pyrroline hydroxycarboxylic acid GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental LC-MS/MS | LC-MS/MS Spectrum - Pyrroline hydroxycarboxylic acid Quattro_QQQ 10V, Positive-QTOF (Annotated) | splash10-001i-9600000000-8e7afe6ced148f01b715 | 2012-07-24 | HMDB team, MONA | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Pyrroline hydroxycarboxylic acid 10V, Positive-QTOF | splash10-03di-3900000000-91b528e579ac4acfac93 | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Pyrroline hydroxycarboxylic acid 20V, Positive-QTOF | splash10-02u0-9400000000-c804fee192934438c532 | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Pyrroline hydroxycarboxylic acid 40V, Positive-QTOF | splash10-014i-9000000000-7da85099ac98a08f5741 | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Pyrroline hydroxycarboxylic acid 10V, Negative-QTOF | splash10-004i-2900000000-ee7c942618fc6b2d94a9 | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Pyrroline hydroxycarboxylic acid 20V, Negative-QTOF | splash10-02e9-9700000000-1d9848f227e64812ada6 | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Pyrroline hydroxycarboxylic acid 40V, Negative-QTOF | splash10-015c-9000000000-0d2b20e1b4dbed83a144 | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Pyrroline hydroxycarboxylic acid 10V, Negative-QTOF | splash10-004i-0900000000-06e1bd2d2793c5f0afaa | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Pyrroline hydroxycarboxylic acid 20V, Negative-QTOF | splash10-0059-9400000000-a84600f4fc13459b9448 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Pyrroline hydroxycarboxylic acid 40V, Negative-QTOF | splash10-0006-9000000000-d2c2d7b1e05ce54b0932 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Pyrroline hydroxycarboxylic acid 10V, Positive-QTOF | splash10-001i-9300000000-c26ff3ebe1bb758e5252 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Pyrroline hydroxycarboxylic acid 20V, Positive-QTOF | splash10-053r-9000000000-97da4a76921516901d46 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Pyrroline hydroxycarboxylic acid 40V, Positive-QTOF | splash10-0pc3-9000000000-8982b409276eceb00f1b | 2021-09-22 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum |
|
|---|
| Biological Properties |
|---|
| Cellular Locations | |
|---|
| Biospecimen Locations | |
|---|
| Tissue Locations | Not Available |
|---|
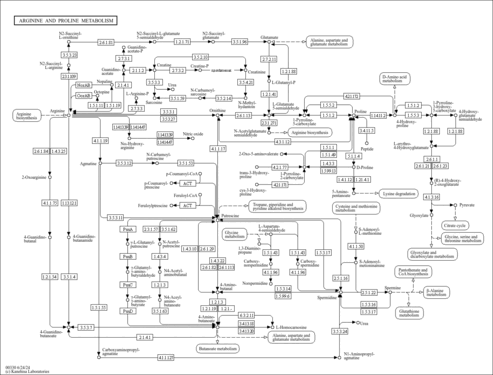
| Pathways | |
|---|
| Normal Concentrations |
|---|
| |
| Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details |
|
|---|
| Abnormal Concentrations |
|---|
| |
| Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Oral cancer | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Female | Breast cancer | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Not Specified | Pancreatic cancer | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Not Specified | Periodontal diseases | | details |
|
|---|
| Associated Disorders and Diseases |
|---|
| Disease References | | Perillyl alcohol administration for cancer treatment |
|---|
- Sugimoto M, Wong DT, Hirayama A, Soga T, Tomita M: Capillary electrophoresis mass spectrometry-based saliva metabolomics identified oral, breast and pancreatic cancer-specific profiles. Metabolomics. 2010 Mar;6(1):78-95. Epub 2009 Sep 10. [PubMed:20300169 ]
| | Pancreatic cancer |
|---|
- Sugimoto M, Wong DT, Hirayama A, Soga T, Tomita M: Capillary electrophoresis mass spectrometry-based saliva metabolomics identified oral, breast and pancreatic cancer-specific profiles. Metabolomics. 2010 Mar;6(1):78-95. Epub 2009 Sep 10. [PubMed:20300169 ]
| | Periodontal disease |
|---|
- Sugimoto M, Wong DT, Hirayama A, Soga T, Tomita M: Capillary electrophoresis mass spectrometry-based saliva metabolomics identified oral, breast and pancreatic cancer-specific profiles. Metabolomics. 2010 Mar;6(1):78-95. Epub 2009 Sep 10. [PubMed:20300169 ]
|
|
|---|
| Associated OMIM IDs | |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| Phenol Explorer Compound ID | Not Available |
|---|
| FooDB ID | FDB022582 |
|---|
| KNApSAcK ID | Not Available |
|---|
| Chemspider ID | 1030 |
|---|
| KEGG Compound ID | C04281 |
|---|
| BioCyc ID | Not Available |
|---|
| BiGG ID | 43511 |
|---|
| Wikipedia Link | Not Available |
|---|
| METLIN ID | 6196 |
|---|
| PubChem Compound | 1059 |
|---|
| PDB ID | Not Available |
|---|
| ChEBI ID | 27391 |
|---|
| Food Biomarker Ontology | Not Available |
|---|
| VMH ID | 1P3H5C |
|---|
| MarkerDB ID | Not Available |
|---|
| Good Scents ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| General References | - Simila S: Hydroxyproline metabolism in type II hyperprolinaemia. Ann Clin Biochem. 1979 Jul;16(4):177-81. [PubMed:533224 ]
- Onenli-Mungan N, Yuksel B, Elkay M, Topaloglu AK, Baykal T, Ozer G: Type II hyperprolinemia: a case report. Turk J Pediatr. 2004 Apr-Jun;46(2):167-9. [PubMed:15214748 ]
- Valle D, Goodman SI, Harris SC, Phang JM: Genetic evidence for a common enzyme catalyzing the second step in the degradation of proline and hydroxyproline. J Clin Invest. 1979 Nov;64(5):1365-70. [PubMed:500817 ]
|
|---|