| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Expected but not Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2022-03-07 02:49:10 UTC |
|---|
| HMDB ID | HMDB0001484 |
|---|
| Secondary Accession Numbers | - HMDB0011665
- HMDB01484
- HMDB11665
|
|---|
| Metabolite Identification |
|---|
| Common Name | Acetoacetyl-CoA |
|---|
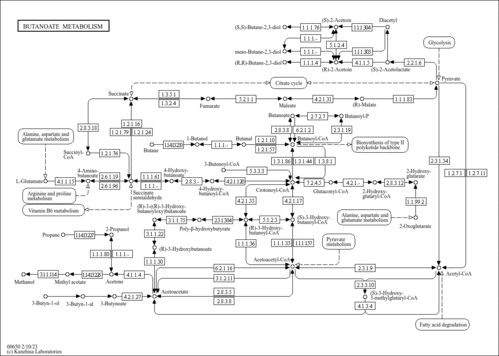
| Description | Acetoacetyl-CoA belongs to the class of organic compounds known as aminopiperidines. Aminopiperidines are compounds containing a piperidine that carries an amino group. Acetoacetyl-CoA is a strong basic compound (based on its pKa). In humans, acetoacetyl-CoA is involved in the metabolic disorder called the short-chain 3-hydroxyacyl-CoA dehydrogenase deficiency (HADH) pathway. Acetoacetyl-CoA is an intermediate in the metabolism of butanoate. It is a substrate for succinyl-CoA:3-ketoacid-coenzyme A transferase, hydroxymethylglutaryl-CoA synthase, short-chain 3-hydroxyacyl-CoA dehydrogenase, peroxisomal bifunctional enzyme, acetyl-CoA acetyltransferase, and 3-ketoacyl-CoA thiolase. |
|---|
| Structure | [H][C@](O)(C(O)=NCCC(O)=NCCSC(=O)CC(C)=O)C(C)(C)COP(O)(=O)OP(O)(=O)OC[C@@]1([H])O[C@@]([H])(N2C=NC3=C(N)N=CN=C23)[C@]([H])(O)[C@]1([H])OP(O)(O)=O InChI=1S/C25H40N7O18P3S/c1-13(33)8-16(35)54-7-6-27-15(34)4-5-28-23(38)20(37)25(2,3)10-47-53(44,45)50-52(42,43)46-9-14-19(49-51(39,40)41)18(36)24(48-14)32-12-31-17-21(26)29-11-30-22(17)32/h11-12,14,18-20,24,36-37H,4-10H2,1-3H3,(H,27,34)(H,28,38)(H,42,43)(H,44,45)(H2,26,29,30)(H2,39,40,41)/t14-,18-,19-,20+,24-/m1/s1 |
|---|
| Synonyms | | Value | Source |
|---|
| 3-Acetoacetyl-CoA | ChEBI | | Acetoacetyl coenzyme A | ChEBI | | S-Acetoacetyl-CoA | ChEBI | | S-Acetoacetyl-coenzym a | ChEBI | | S-Acetoacetyl-coenzyme A | ChEBI | | Acetoacetyl CoA | MeSH |
|
|---|
| Chemical Formula | C25H40N7O18P3S |
|---|
| Average Molecular Weight | 851.607 |
|---|
| Monoisotopic Molecular Weight | 851.136337737 |
|---|
| IUPAC Name | {[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-2-({[hydroxy({hydroxy[(3R)-3-hydroxy-2,2-dimethyl-3-{[2-({2-[(3-oxobutanoyl)sulfanyl]ethyl}carbamoyl)ethyl]carbamoyl}propoxy]phosphoryl}oxy)phosphoryl]oxy}methyl)oxolan-3-yl]oxy}phosphonic acid |
|---|
| Traditional Name | acetoacetyl-coa |
|---|
| CAS Registry Number | 1420-36-6 |
|---|
| SMILES | CC(=O)CC(=O)SCCNC(=O)CCNC(=O)[C@H](O)C(C)(C)COP(O)(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP(O)(O)=O)N1C=NC2=C1N=CN=C2N |
|---|
| InChI Identifier | InChI=1S/C25H40N7O18P3S/c1-13(33)8-16(35)54-7-6-27-15(34)4-5-28-23(38)20(37)25(2,3)10-47-53(44,45)50-52(42,43)46-9-14-19(49-51(39,40)41)18(36)24(48-14)32-12-31-17-21(26)29-11-30-22(17)32/h11-12,14,18-20,24,36-37H,4-10H2,1-3H3,(H,27,34)(H,28,38)(H,42,43)(H,44,45)(H2,26,29,30)(H2,39,40,41)/t14-,18-,19-,20+,24-/m1/s1 |
|---|
| InChI Key | OJFDKHTZOUZBOS-CITAKDKDSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as aminopiperidines. Aminopiperidines are compounds containing a piperidine that carries an amino group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Piperidines |
|---|
| Sub Class | Aminopiperidines |
|---|
| Direct Parent | Aminopiperidines |
|---|
| Alternative Parents | |
|---|
| Substituents | - 4-aminopiperidine
- Tertiary aliphatic amine
- Tertiary amine
- Azacycle
- Secondary amine
- Secondary aliphatic amine
- Organic nitrogen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organonitrogen compound
- Amine
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Not Available |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Kovats Retention IndicesUnderivatized |
|---|
| MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetoacetyl-CoA n/a 59V, negative-QTOF | splash10-000i-0004009000-9559fa356f1bae03b7ca | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetoacetyl-CoA n/a 59V, negative-QTOF | splash10-0a4i-0269300000-d5574d3fd84812c0057d | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetoacetyl-CoA n/a 59V, negative-QTOF | splash10-05n1-0009600000-a29b94fc4d454c655bab | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetoacetyl-CoA n/a 59V, negative-QTOF | splash10-000i-0001209000-21979fdba010c3871c5a | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetoacetyl-CoA Orbitrap 42V, negative-QTOF | splash10-0udi-0000000090-aa1f97ad009df4ad9439 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetoacetyl-CoA Orbitrap 57V, negative-QTOF | splash10-0udi-0000100490-97b56edeb43a5d82ba85 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetoacetyl-CoA Orbitrap 71V, negative-QTOF | splash10-014i-0000910830-9d7a9bb5d2817f98fbf1 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetoacetyl-CoA Orbitrap 93V, negative-QTOF | splash10-0ar9-0101901000-ee3c1d5ebb3142f3c5af | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetoacetyl-CoA Orbitrap 115V, negative-QTOF | splash10-0a6r-5524900000-2d745cfa452462059e2c | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetoacetyl-CoA Orbitrap 136V, negative-QTOF | splash10-056r-9513200000-037d88669e973d0dff1c | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetoacetyl-CoA Orbitrap 158V, negative-QTOF | splash10-004i-9400000000-fca9f3020e4e007e8164 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetoacetyl-CoA Orbitrap 187V, negative-QTOF | splash10-004i-9300000000-13ad94b2682d99b6234d | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetoacetyl-CoA Orbitrap 216V, negative-QTOF | splash10-004i-9200000000-32dfa0b8d343e2eb7b7b | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetoacetyl-CoA Orbitrap 260V, negative-QTOF | splash10-004i-9100000000-6461ef6280ce0db781b2 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetoacetyl-CoA n/a 59V, negative-QTOF | splash10-014i-0000000900-727bb0c3c925e7bbeba4 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetoacetyl-CoA n/a 59V, negative-QTOF | splash10-00di-0190000000-0e093fb5398057b2093d | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetoacetyl-CoA n/a 59V, negative-QTOF | splash10-0a4i-0900000000-b4c90f8c858e783159b8 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetoacetyl-CoA n/a 59V, negative-QTOF | splash10-0udl-0018900000-91aaf6888a10aac16f57 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetoacetyl-CoA n/a 59V, negative-QTOF | splash10-00xr-0092000000-2dd3c44dfb7a39ee9a59 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Acetoacetyl-CoA 10V, Positive-QTOF | splash10-000i-1912000130-163ca7231778bddd9739 | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Acetoacetyl-CoA 20V, Positive-QTOF | splash10-000i-0913000000-4f4f26abcf9b0d9f285e | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Acetoacetyl-CoA 40V, Positive-QTOF | splash10-000i-2911000000-cc9acdeb298fa6ebbdb7 | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Acetoacetyl-CoA 10V, Negative-QTOF | splash10-001i-9730140350-cefdc8a0886f8e9e2b86 | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Acetoacetyl-CoA 20V, Negative-QTOF | splash10-001i-5910110010-51e3282fd98f872b9ff5 | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Acetoacetyl-CoA 40V, Negative-QTOF | splash10-057i-6900100000-4a6e405bfbd488ea01d4 | 2015-09-15 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum |
|
|---|