| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected and Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2023-02-21 17:15:47 UTC |
|---|
| HMDB ID | HMDB0001553 |
|---|
| Secondary Accession Numbers | - HMDB0013210
- HMDB01553
- HMDB13210
|
|---|
| Metabolite Identification |
|---|
| Common Name | 2-Oxo-4-methylthiobutanoic acid |
|---|
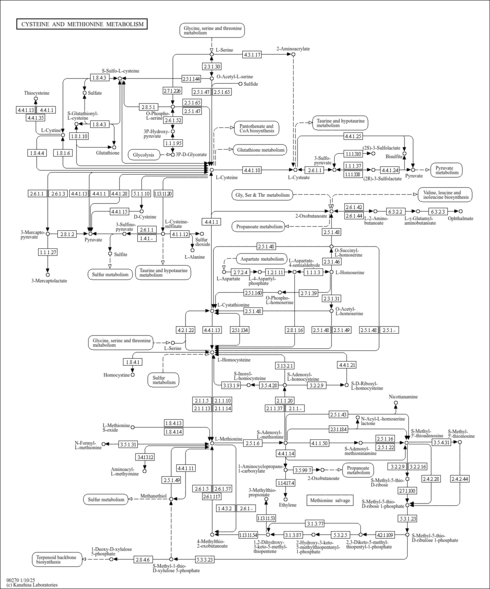
| Description | 2-oxo-4-methylthiobutanoate, also known as 2-keto-4-methylthiobutyric acid, 2-keto-4-methylthiobutyrate or 4-(methylsulfanyl)-2-oxobutanoic acid, is a member of the class of compounds known as thia- fatty acids. Thia-fatty acids are fatty acid derivatives obtained by insertion of a sulfur atom at specific positions in the chain. Thus, 2-oxo-4-methylthiobutanoate is a fatty acid lipid molecule. 2-oxo-4-methylthiobutanoate is slightly soluble (in water) and a weakly acidic compound (based on its pKa). 2-oxo-4-methylthiobutanoate can be synthesized from L-methionine and butyric acid. 2-oxo-4-methylthiobutanoate can also be synthesized into S-adenosyl-4-methylthio-2-oxobutanoic acid. 2-oxo-4-methylthiobutanoate can be found in a number of food items such as cloves, highbush blueberries, common beets, and cashew nuts. 2-oxo-4-methylthiobutanoate can be found in urine. Within the cell, 2-oxo-4-methylthiobutanoate is primarily located in the cytoplasm and in the membrane. 2-oxo-4-methylthiobutanoate has been found in all living species, from bacteria to humans. In humans, 2-oxo-4-methylthiobutanoate is found to be involved in several metabolic disorders, some of those are S-adenosylhomocysteine (SAH) hydrolase deficiency, methylenetetrahydrofolate reductase deficiency (MTHFRD), methionine adenosyltransferase deficiency, and glycine N-methyltransferase deficiency. 4-Methylthio-2-oxobutanoic acid is the direct precursor of methional, which is a potent inducer of apoptosis in a BAF3 murine lymphoid cell line which is interleukin-3 (IL3)-dependent (PMID: 7848263 ). |
|---|
| Structure | InChI=1S/C5H8O3S/c1-9-3-2-4(6)5(7)8/h2-3H2,1H3,(H,7,8) |
|---|
| Synonyms | | Value | Source |
|---|
| 2-Keto-4-methylthiobutyric acid | ChEBI | | 2-oxo-4-Methylthiobutanoate | ChEBI | | 2-Oxomethionine | ChEBI | | 4-(METHYLsulfanyl)-2-oxobutanoIC ACID | ChEBI | | 4-Methylthio-2-oxobutanoate | ChEBI | | alpha-oxo-gamma-Methylthiobutyric acid | ChEBI | | 4-(Methylsulfanyl)-2-oxobutanoate | Kegg | | 2-Keto-4-methylthiobutyrate | Generator | | 4-(METHYLsulphanyl)-2-oxobutanoate | Generator | | 4-(METHYLsulphanyl)-2-oxobutanoic acid | Generator | | 4-Methylthio-2-oxobutanoic acid | Generator | | a-oxo-g-Methylthiobutyrate | Generator | | a-oxo-g-Methylthiobutyric acid | Generator | | alpha-oxo-gamma-Methylthiobutyrate | Generator | | Α-oxo-γ-methylthiobutyrate | Generator | | Α-oxo-γ-methylthiobutyric acid | Generator | | 2-Ketomethiobutyrate | HMDB | | 4-Methylthio-2-ketobutanoate | HMDB | | 4-Methylthio-2-ketobutanoic acid | HMDB | | 4-Methylthio-2-ketobutyrate | HMDB | | 4-Methylthio-2-oxobutyrate | HMDB | | alpha-Keto-methiolbutyric acid | HMDB | | Keto-4-methylthiobutyrate | HMDB | | Ketomethiobutyrate | HMDB | | Ketomethiobutyric acid | HMDB | | KMTB | HMDB | | Methylthiobutyrate | HMDB | | Methylthiobutyric acid | HMDB | | 2-Keto-4-methylthiobutanoic acid | HMDB | | 2-Keto-4-thiomethylbutyrate | HMDB | | 2-oxo-4-Thiomethylbutyric acid | HMDB | | alpha-Keto-gamma-methiolbutyrate | HMDB | | alpha-Ketomethionine | HMDB | | alpha-Oxomethionine | HMDB | | 2-Keto-4-methylthiobutyric acid, monosodium salt | HMDB | | 2-Ketothiomethylbutyric acid | HMDB | | 4-Methylthio-2-oxobutyric acid | HMDB | | S-Methyl-alpha-ketobutyric acid | HMDB | | 2-Keto-4-methylthiobutyric acid, calcium salt | HMDB | | 4-Methylthio-2-ketobutyric acid | HMDB | | gamma-Methiol-keto-butyric acid | HMDB | | 4-Methylmercapto-2-oxobutyrate | HMDB | | 2-oxo-4-Methylthiobutanoic acid | KEGG |
|
|---|
| Chemical Formula | C5H8O3S |
|---|
| Average Molecular Weight | 148.18 |
|---|
| Monoisotopic Molecular Weight | 148.019414812 |
|---|
| IUPAC Name | 4-(methylsulfanyl)-2-oxobutanoic acid |
|---|
| Traditional Name | 2-oxo-4-thiomethylbutyric acid |
|---|
| CAS Registry Number | 583-92-6 |
|---|
| SMILES | CSCCC(=O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C5H8O3S/c1-9-3-2-4(6)5(7)8/h2-3H2,1H3,(H,7,8) |
|---|
| InChI Key | SXFSQZDSUWACKX-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as thia fatty acids. These are fatty acid derivatives obtained by insertion of a sulfur atom at specific positions in the chain. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acids and conjugates |
|---|
| Direct Parent | Thia fatty acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Short-chain keto acid
- Thia fatty acid
- Alpha-keto acid
- Keto acid
- Alpha-hydroxy ketone
- Ketone
- Carboxylic acid derivative
- Carboxylic acid
- Dialkylthioether
- Monocarboxylic acid or derivatives
- Sulfenyl compound
- Thioether
- Hydrocarbon derivative
- Organic oxide
- Carbonyl group
- Organooxygen compound
- Organosulfur compound
- Organic oxygen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Not Available |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| 2-Oxo-4-methylthiobutanoic acid,1TMS,isomer #1 | CSCCC(=O)C(=O)O[Si](C)(C)C | 1362.1 | Semi standard non polar | 33892256 | | 2-Oxo-4-methylthiobutanoic acid,1TMS,isomer #2 | CSCC=C(O[Si](C)(C)C)C(=O)O | 1461.5 | Semi standard non polar | 33892256 | | 2-Oxo-4-methylthiobutanoic acid,2TMS,isomer #1 | CSCC=C(O[Si](C)(C)C)C(=O)O[Si](C)(C)C | 1505.4 | Semi standard non polar | 33892256 | | 2-Oxo-4-methylthiobutanoic acid,2TMS,isomer #1 | CSCC=C(O[Si](C)(C)C)C(=O)O[Si](C)(C)C | 1466.5 | Standard non polar | 33892256 | | 2-Oxo-4-methylthiobutanoic acid,2TMS,isomer #1 | CSCC=C(O[Si](C)(C)C)C(=O)O[Si](C)(C)C | 1638.6 | Standard polar | 33892256 | | 2-Oxo-4-methylthiobutanoic acid,1TBDMS,isomer #1 | CSCCC(=O)C(=O)O[Si](C)(C)C(C)(C)C | 1609.3 | Semi standard non polar | 33892256 | | 2-Oxo-4-methylthiobutanoic acid,1TBDMS,isomer #2 | CSCC=C(O[Si](C)(C)C(C)(C)C)C(=O)O | 1699.0 | Semi standard non polar | 33892256 | | 2-Oxo-4-methylthiobutanoic acid,2TBDMS,isomer #1 | CSCC=C(O[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C | 1972.1 | Semi standard non polar | 33892256 | | 2-Oxo-4-methylthiobutanoic acid,2TBDMS,isomer #1 | CSCC=C(O[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C | 1880.3 | Standard non polar | 33892256 | | 2-Oxo-4-methylthiobutanoic acid,2TBDMS,isomer #1 | CSCC=C(O[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C | 1910.7 | Standard polar | 33892256 |
|
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental GC-MS | GC-MS Spectrum - 2-Oxo-4-methylthiobutanoic acid GC-MS (1 MEOX; 1 TMS) | splash10-0f79-9620000000-a37f1649aaa00fe355f6 | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - 2-Oxo-4-methylthiobutanoic acid GC-MS (1 MEOX; 1 TMS) | splash10-0uyr-7940000000-be5c411217da093f2c8a | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - 2-Oxo-4-methylthiobutanoic acid GC-MS (Non-derivatized) | splash10-0f79-9620000000-a37f1649aaa00fe355f6 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - 2-Oxo-4-methylthiobutanoic acid GC-MS (Non-derivatized) | splash10-0uyr-7940000000-be5c411217da093f2c8a | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 2-Oxo-4-methylthiobutanoic acid GC-MS (Non-derivatized) - 70eV, Positive | splash10-0005-9200000000-1471d6fe57ac965a873d | 2016-09-22 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 2-Oxo-4-methylthiobutanoic acid GC-MS (1 TMS) - 70eV, Positive | splash10-0g4j-9400000000-16ddd3b5c053991fac9a | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 2-Oxo-4-methylthiobutanoic acid GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental LC-MS/MS | LC-MS/MS Spectrum - 2-Oxo-4-methylthiobutanoic acid LC-ESI-QTOF 35V, positive-QTOF | splash10-0a6t-0900000000-ed86a80611ad68e921e2 | 2020-07-21 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 2-Oxo-4-methylthiobutanoic acid LC-ESI-QFT , negative-QTOF | splash10-0002-9000000000-9b1238b1c990e180a476 | 2020-07-21 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 2-Oxo-4-methylthiobutanoic acid LC-ESI-QTOF 35V, negative-QTOF | splash10-0002-9000000000-a0fb6b922f7482546f1f | 2020-07-21 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 2-Oxo-4-methylthiobutanoic acid Orbitrap 0V, negative-QTOF | splash10-0002-1900000000-f3facf4268a57538266c | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 2-Oxo-4-methylthiobutanoic acid Orbitrap 0V, negative-QTOF | splash10-0002-2900000000-71e4f52b95b529acc1a3 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 2-Oxo-4-methylthiobutanoic acid Orbitrap 0V, negative-QTOF | splash10-0002-5900000000-c6325fbf5479dbaa355c | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 2-Oxo-4-methylthiobutanoic acid Orbitrap 0V, negative-QTOF | splash10-0002-7900000000-bccb79b226dccd29e36f | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 2-Oxo-4-methylthiobutanoic acid Orbitrap 1V, negative-QTOF | splash10-0002-9700000000-8e6b66c2a5dd967a9539 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 2-Oxo-4-methylthiobutanoic acid Orbitrap 1V, negative-QTOF | splash10-0002-9500000000-dee1d47be79038a62ac8 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 2-Oxo-4-methylthiobutanoic acid Orbitrap 1V, negative-QTOF | splash10-0002-9200000000-184f804af473361e56d4 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 2-Oxo-4-methylthiobutanoic acid Orbitrap 2V, negative-QTOF | splash10-0002-9200000000-9f652bc59797907a90e8 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 2-Oxo-4-methylthiobutanoic acid Orbitrap 2V, negative-QTOF | splash10-0002-9100000000-2924be77506cc359576f | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 2-Oxo-4-methylthiobutanoic acid Orbitrap 3V, negative-QTOF | splash10-0002-9000000000-ac83262a096530d8343f | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 2-Oxo-4-methylthiobutanoic acid Orbitrap 3V, negative-QTOF | splash10-0002-9000000000-12841f2d918636eb3ba8 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 2-Oxo-4-methylthiobutanoic acid n/a 10V, negative-QTOF | splash10-0002-9000000000-beb5b9021683b7e71f41 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 2-Oxo-4-methylthiobutanoic acid n/a 10V, negative-QTOF | splash10-0a4i-9000000000-5c4795e954d5c3e9b74e | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 2-Oxo-4-methylthiobutanoic acid Orbitrap 0V, positive-QTOF | splash10-0006-0900000000-a7b6fa585a128c4bbf25 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 2-Oxo-4-methylthiobutanoic acid Orbitrap 1V, positive-QTOF | splash10-0006-0900000000-5d4054fa9cdf6414108d | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 2-Oxo-4-methylthiobutanoic acid Orbitrap 2V, positive-QTOF | splash10-0006-0900000000-70154862c61d3d6ece44 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 2-Oxo-4-methylthiobutanoic acid 10V, Positive-QTOF | splash10-0f8a-1900000000-ed36a40a48bf904312f4 | 2015-05-27 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 2-Oxo-4-methylthiobutanoic acid 20V, Positive-QTOF | splash10-0zir-9700000000-7bbe813aa31ceba0b525 | 2015-05-27 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 2-Oxo-4-methylthiobutanoic acid 40V, Positive-QTOF | splash10-0a6r-9100000000-89ba14ad10b374e8daab | 2015-05-27 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 2-Oxo-4-methylthiobutanoic acid 10V, Negative-QTOF | splash10-0002-9200000000-b9179ec180a89222c147 | 2015-05-27 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 2-Oxo-4-methylthiobutanoic acid 20V, Negative-QTOF | splash10-0002-9100000000-63d77e1e6b7bea5353fe | 2015-05-27 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 2-Oxo-4-methylthiobutanoic acid 40V, Negative-QTOF | splash10-0002-9000000000-31b5d7804293c55c928e | 2015-05-27 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum |
IR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M-H]-) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+H]+) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+Na]+) | 2023-02-03 | FELIX lab | View Spectrum |
|
|---|