| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Expected but not Quantified |
|---|
| Creation Date | 2006-08-12 20:13:38 UTC |
|---|
| Update Date | 2021-09-14 15:45:00 UTC |
|---|
| HMDB ID | HMDB0003403 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Amylose |
|---|
| Description | Amylose is defined as a linear molecule of (1→4) linked alpha-D-glucopyranosyl units, but it is today well established that some molecules are slightly branched by (1→6)-alpha-linkages. The oldest criteria for linearity consisted in the susceptibility of the molecule to complete hydrolysis by beta-amylase. This enzyme splits the (1→4) bonds from the non-reducing end of a chain releasing beta-maltosyl units, but cannot cleave the (1→6) bonds. When degraded by pure beta-amylase, linear macromolecules are completely converted into maltose, whereas branched chains give also one beta-limit dextrin consisting of the remaining inner core polysaccharide structure with its outer chains recessed. Starches of different botanical origins possess different granular sizes, morphology, polymorphism and enzyme digestibility. These characteristics are related to the chemical structures of the amylopectin and amylose and how they are arranged in the starch granule (PMID 9730163 ). |
|---|
| Structure | CO[C@H]1O[C@H](CO)[C@@H](O[C@H]2O[C@H](CO)[C@@H](OC)[C@H](O)[C@H]2O)[C@H](O)[C@H]1O InChI=1S/C14H26O11/c1-21-11-5(3-15)24-14(10(20)7(11)17)25-12-6(4-16)23-13(22-2)9(19)8(12)18/h5-20H,3-4H2,1-2H3/t5-,6-,7-,8-,9-,10-,11-,12-,13+,14-/m1/s1 |
|---|
| Synonyms | | Value | Source |
|---|
| (1,4-alpha-D-Glucosyl)N | HMDB | | (1,4-alpha-D-Glucosyl)n+1 | HMDB | | (1,4-alpha-D-Glucosyl)N-1 | HMDB | | (1,4-alpha-delta-Glucosyl)N | HMDB | | (1,4-alpha-delta-Glucosyl)n+1 | HMDB | | (1,4-alpha-delta-Glucosyl)N-1 | HMDB | | 1,4-alpha-D-Glucan | HMDB | | 1,4-alpha-delta-Glucan | HMDB | | 4-{(1,4)-alpha-D-glucosyl}(N-1)-D-glucose | HMDB | | 4-{(1,4)-alpha-delta-glucosyl}(N-1)-delta-glucose | HMDB | | Amylose chain | HMDB | | a-D-Mannose | HMDB | | Α-D-mannose | HMDB |
|
|---|
| Chemical Formula | (C12H20O11)nC2H6 |
|---|
| Average Molecular Weight | Not Available |
|---|
| Monoisotopic Molecular Weight | Not Available |
|---|
| IUPAC Name | Not Available |
|---|
| Traditional Name | Not Available |
|---|
| CAS Registry Number | 9005-82-7 |
|---|
| SMILES | CO[C@H]1O[C@H](CO)[C@@H](O[C@H]2O[C@H](CO)[C@@H](OC)[C@H](O)[C@H]2O)[C@H](O)[C@H]1O |
|---|
| InChI Identifier | InChI=1S/C14H26O11/c1-21-11-5(3-15)24-14(10(20)7(11)17)25-12-6(4-16)23-13(22-2)9(19)8(12)18/h5-20H,3-4H2,1-2H3/t5-,6-,7-,8-,9-,10-,11-,12-,13+,14-/m1/s1 |
|---|
| InChI Key | PTHCMJGKKRQCBF-OLYDTGNASA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as o-glycosyl compounds. These are glycoside in which a sugar group is bonded through one carbon to another group via a O-glycosidic bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | O-glycosyl compounds |
|---|
| Alternative Parents | |
|---|
| Substituents | - O-glycosyl compound
- Disaccharide
- Oxane
- Secondary alcohol
- Oxacycle
- Organoheterocyclic compound
- Ether
- Dialkyl ether
- Acetal
- Hydrocarbon derivative
- Primary alcohol
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | Not Available |
|---|
| Process | |
|---|
| Role | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Not Available |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Retention Times Underivatized| Chromatographic Method | Retention Time | Reference |
|---|
| Measured using a Waters Acquity ultraperformance liquid chromatography (UPLC) ethylene-bridged hybrid (BEH) C18 column (100 mm × 2.1 mm; 1.7 μmparticle diameter). Predicted by Afia on May 17, 2022. Predicted by Afia on May 17, 2022. | 1.75 minutes | 32390414 | | Predicted by Siyang on May 30, 2022 | 9.9223 minutes | 33406817 | | Predicted by Siyang using ReTip algorithm on June 8, 2022 | 7.74 minutes | 32390414 | | AjsUoB = Accucore 150 Amide HILIC with 10mM Ammonium Formate, 0.1% Formic Acid | 264.2 seconds | 40023050 | | Fem_Long = Waters ACQUITY UPLC HSS T3 C18 with Water:MeOH and 0.1% Formic Acid | 1124.5 seconds | 40023050 | | Fem_Lipids = Ascentis Express C18 with (60:40 water:ACN):(90:10 IPA:ACN) and 10mM NH4COOH + 0.1% Formic Acid | 218.6 seconds | 40023050 | | Life_Old = Waters ACQUITY UPLC BEH C18 with Water:(20:80 acetone:ACN) and 0.1% Formic Acid | 61.2 seconds | 40023050 | | Life_New = RP Waters ACQUITY UPLC HSS T3 C18 with Water:(30:70 MeOH:ACN) and 0.1% Formic Acid | 179.6 seconds | 40023050 | | RIKEN = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 51.3 seconds | 40023050 | | Eawag_XBridgeC18 = XBridge C18 3.5u 2.1x50 mm with Water:MeOH and 0.1% Formic Acid | 288.3 seconds | 40023050 | | BfG_NTS_RP1 =Agilent Zorbax Eclipse Plus C18 (2.1 mm x 150 mm, 3.5 um) with Water:ACN and 0.1% Formic Acid | 289.2 seconds | 40023050 | | HILIC_BDD_2 = Merck SeQuant ZIC-HILIC with ACN(0.1% formic acid):water(16 mM ammonium formate) | 688.0 seconds | 40023050 | | UniToyama_Atlantis = RP Waters Atlantis T3 (2.1 x 150 mm, 5 um) with ACN:Water and 0.1% Formic Acid | 622.1 seconds | 40023050 | | BDD_C18 = Hypersil Gold 1.9µm C18 with Water:ACN and 0.1% Formic Acid | 94.2 seconds | 40023050 | | UFZ_Phenomenex = Kinetex Core-Shell C18 2.6 um, 3.0 x 100 mm, Phenomenex with Water:MeOH and 0.1% Formic Acid | 906.5 seconds | 40023050 | | SNU_RIKEN_POS = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 222.6 seconds | 40023050 | | RPMMFDA = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 227.3 seconds | 40023050 | | MTBLS87 = Merck SeQuant ZIC-pHILIC column with ACN:Water and :ammonium carbonate | 491.2 seconds | 40023050 | | KI_GIAR_zic_HILIC_pH2_7 = Merck SeQuant ZIC-HILIC with ACN:Water and 0.1% FA | 363.1 seconds | 40023050 | | Meister zic-pHILIC pH9.3 = Merck SeQuant ZIC-pHILIC column with ACN:Water 5mM NH4Ac pH9.3 and 5mM ammonium acetate in water | 242.1 seconds | 40023050 |
Predicted Kovats Retention IndicesNot Available |
|---|
| Spectra |
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - Amylose GC-MS (Non-derivatized) - 70eV, Positive | splash10-0k9i-3329000000-2b7bd69033e8a4fea64a | 2017-11-06 | Wishart Lab | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-0229-9000000000-3cd18e57966112492f50 | 2018-05-25 | Not Available | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental LC-MS/MS | LC-MS/MS Spectrum - Amylose Quattro_QQQ 10V, Positive-QTOF (Annotated) | splash10-0udi-0900000000-5eb2b079a3b131ab742e | 2012-07-25 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Amylose Quattro_QQQ 25V, Positive-QTOF (Annotated) | splash10-0fb9-9700000000-8631a9ae75f1a201adc7 | 2012-07-25 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Amylose Quattro_QQQ 40V, Positive-QTOF (Annotated) | splash10-004i-9000000000-9b80204fe6afbb2eaf15 | 2012-07-25 | HMDB team, MONA | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Amylose 10V, Positive-QTOF | splash10-01q9-0900000000-865ff3c21e042be41194 | 2018-11-27 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Amylose 20V, Positive-QTOF | splash10-03ea-1900000000-ff3096b7f2c9d1141b1f | 2018-11-27 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Amylose 40V, Positive-QTOF | splash10-0007-9100000000-c4b6f8b4217029a276d9 | 2018-11-27 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Amylose 10V, Positive-QTOF | splash10-00di-0609000000-0dcb8fef7c013777f12e | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Amylose 20V, Positive-QTOF | splash10-004j-1900000000-3ea35321e04f2cba1d3a | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Amylose 40V, Positive-QTOF | splash10-0bvj-9710000000-bb5f9bcfdec86cf6b78a | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Amylose 10V, Negative-QTOF | splash10-014i-0209000000-59d4bec3247cdead5672 | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Amylose 20V, Negative-QTOF | splash10-016r-4948000000-7a4b110014b05330fed4 | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Amylose 40V, Negative-QTOF | splash10-0596-9310000000-b79abc5ca8f54ee5ef9c | 2021-09-24 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Experimental 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, 100%_DMSO, experimental) | 2012-12-05 | Wishart Lab | View Spectrum |
|
|---|
| Biological Properties |
|---|
| Cellular Locations | Not Available |
|---|
| Biospecimen Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
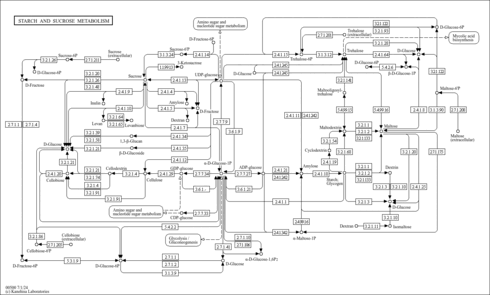
| Pathways | |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| Associated Disorders and Diseases |
|---|
| Disease References | None |
|---|
| Associated OMIM IDs | None |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| Phenol Explorer Compound ID | Not Available |
|---|
| FooDB ID | FDB001130 |
|---|
| KNApSAcK ID | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| KEGG Compound ID | C00718 |
|---|
| BioCyc ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| Wikipedia Link | Amylose |
|---|
| METLIN ID | 6917 |
|---|
| PubChem Compound | 53477771 |
|---|
| PDB ID | Not Available |
|---|
| ChEBI ID | 28102 |
|---|
| Food Biomarker Ontology | Not Available |
|---|
| VMH ID | Not Available |
|---|
| MarkerDB ID | Not Available |
|---|
| Good Scents ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Breitinger, Hans-Georg. Synthesis and characterization of 2,3-di-O-alkylated amyloses: Hydrophobic substitution destabilizes helical conformation. Biopolymers (2003), 69(3), 301-310. |
|---|
| Material Safety Data Sheet (MSDS) | Download (PDF) |
|---|
| General References | - Topping DL, Gooden JM, Brown IL, Biebrick DA, McGrath L, Trimble RP, Choct M, Illman RJ: A high amylose (amylomaize) starch raises proximal large bowel starch and increases colon length in pigs. J Nutr. 1997 Apr;127(4):615-22. [PubMed:9109613 ]
- Klein B, Foreman JA: Amylolysis of a chromogenic substrate, Cibachron Blue F3GA-amylose: kinetics and mechanism. Clin Chem. 1980 Feb;26(2):250-3. [PubMed:6153298 ]
- Langkilde AM, Ekwall H, Bjorck I, Asp NG, Andersson H: Retrograded high-amylose corn starch reduces cholic acid excretion from the small bowel in ileostomy subjects. Eur J Clin Nutr. 1998 Nov;52(11):790-5. [PubMed:9846590 ]
- Traub WH: Studies on neutralization of human serum bactericidal activity by sodium amylosulfate. J Clin Microbiol. 1977 Aug;6(2):128-31. [PubMed:330560 ]
- Buleon A, Colonna P, Planchot V, Ball S: Starch granules: structure and biosynthesis. Int J Biol Macromol. 1998 Aug;23(2):85-112. [PubMed:9730163 ]
|
|---|