| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Expected but not Quantified |
|---|
| Creation Date | 2006-08-12 21:36:15 UTC |
|---|
| Update Date | 2023-02-21 17:16:42 UTC |
|---|
| HMDB ID | HMDB0003470 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | N-Formyl-L-glutamic acid |
|---|
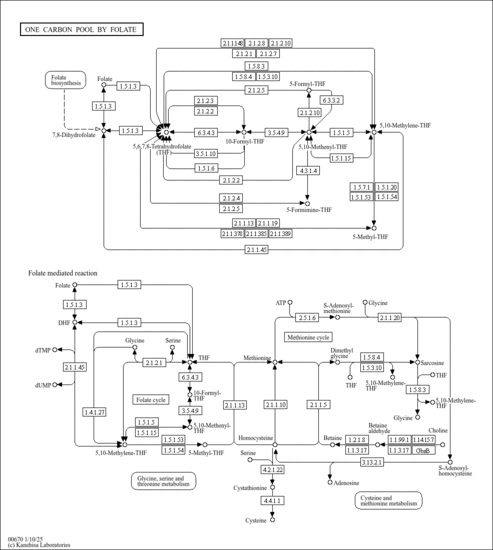
| Description | N-Formyl-L-glutamic acid, also known as N-formylglutamate, ion(2-) or N-formyl-L-glutamate, belongs to the class of organic compounds known as glutamic acid and derivatives. Glutamic acid and derivatives are compounds containing glutamic acid or a derivative thereof resulting from reaction of glutamic acid at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. N-Formyl-L-glutamic acid exists in all living organisms, ranging from bacteria to humans. In humans, N-formyl-L-glutamic acid is involved in the folate malabsorption, hereditary pathway. N-Formyl-L-glutamic acid has been detected, but not quantified in, a few different foods, such as anatidaes (Anatidae), chickens (Gallus gallus), and domestic pigs (Sus scrofa domestica). This could make N-formyl-L-glutamic acid a potential biomarker for the consumption of these foods. N-Formyl-L-glutamic acid is a primary metabolite. Primary metabolites are metabolically or physiologically essential metabolites. They are directly involved in an organism’s growth, development or reproduction. Based on a literature review very few articles have been published on N-Formyl-L-glutamic acid. |
|---|
| Structure | OC(=O)CC[C@H](NC=O)C(O)=O InChI=1S/C6H9NO5/c8-3-7-4(6(11)12)1-2-5(9)10/h3-4H,1-2H2,(H,7,8)(H,9,10)(H,11,12)/t4-/m0/s1 |
|---|
| Synonyms | | Value | Source |
|---|
| (2S)-2-(Formylamino)pentanedioic acid | ChEBI | | N-Formyl-L-glutamate | ChEBI | | (2S)-2-(Formylamino)pentanedioate | Generator | | N-Formylglutamate, ion(2-) | MeSH | | N-Formylglutamate | MeSH | | (2S)-2-Formamidopentanedioate | HMDB | | (2S)-2-Formamidopentanedioic acid | HMDB |
|
|---|
| Chemical Formula | C6H9NO5 |
|---|
| Average Molecular Weight | 175.1394 |
|---|
| Monoisotopic Molecular Weight | 175.048072403 |
|---|
| IUPAC Name | (2S)-2-formamidopentanedioic acid |
|---|
| Traditional Name | N-formyl-L-glutamic acid |
|---|
| CAS Registry Number | 1681-96-5 |
|---|
| SMILES | OC(=O)CC[C@H](NC=O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C6H9NO5/c8-3-7-4(6(11)12)1-2-5(9)10/h3-4H,1-2H2,(H,7,8)(H,9,10)(H,11,12)/t4-/m0/s1 |
|---|
| InChI Key | ADZLWSMFHHHOBV-BYPYZUCNSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as glutamic acid and derivatives. Glutamic acid and derivatives are compounds containing glutamic acid or a derivative thereof resulting from reaction of glutamic acid at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Glutamic acid and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Glutamic acid or derivatives
- N-acyl-l-alpha-amino acid
- N-formyl-alpha-amino acid
- N-formyl-alpha amino acid or derivatives
- Dicarboxylic acid or derivatives
- Fatty acid
- Secondary carboxylic acid amide
- Carboxamide group
- Carboxylic acid
- Organopnictogen compound
- Carbonyl group
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Not Available |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| N-Formyl-L-glutamic acid,1TMS,isomer #1 | C[Si](C)(C)OC(=O)CC[C@H](NC=O)C(=O)O | 1654.9 | Semi standard non polar | 33892256 | | N-Formyl-L-glutamic acid,1TMS,isomer #2 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC=O | 1641.2 | Semi standard non polar | 33892256 | | N-Formyl-L-glutamic acid,1TMS,isomer #3 | C[Si](C)(C)N(C=O)[C@@H](CCC(=O)O)C(=O)O | 1722.2 | Semi standard non polar | 33892256 | | N-Formyl-L-glutamic acid,2TMS,isomer #1 | C[Si](C)(C)OC(=O)CC[C@H](NC=O)C(=O)O[Si](C)(C)C | 1715.7 | Semi standard non polar | 33892256 | | N-Formyl-L-glutamic acid,2TMS,isomer #2 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O)N(C=O)[Si](C)(C)C | 1761.5 | Semi standard non polar | 33892256 | | N-Formyl-L-glutamic acid,2TMS,isomer #3 | C[Si](C)(C)OC(=O)[C@H](CCC(=O)O)N(C=O)[Si](C)(C)C | 1769.8 | Semi standard non polar | 33892256 | | N-Formyl-L-glutamic acid,3TMS,isomer #1 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O[Si](C)(C)C)N(C=O)[Si](C)(C)C | 1789.3 | Semi standard non polar | 33892256 | | N-Formyl-L-glutamic acid,3TMS,isomer #1 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O[Si](C)(C)C)N(C=O)[Si](C)(C)C | 1770.0 | Standard non polar | 33892256 | | N-Formyl-L-glutamic acid,3TMS,isomer #1 | C[Si](C)(C)OC(=O)CC[C@@H](C(=O)O[Si](C)(C)C)N(C=O)[Si](C)(C)C | 2015.7 | Standard polar | 33892256 | | N-Formyl-L-glutamic acid,1TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@H](NC=O)C(=O)O | 1905.3 | Semi standard non polar | 33892256 | | N-Formyl-L-glutamic acid,1TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OC(=O)[C@H](CCC(=O)O)NC=O | 1874.2 | Semi standard non polar | 33892256 | | N-Formyl-L-glutamic acid,1TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)N(C=O)[C@@H](CCC(=O)O)C(=O)O | 1945.1 | Semi standard non polar | 33892256 | | N-Formyl-L-glutamic acid,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@H](NC=O)C(=O)O[Si](C)(C)C(C)(C)C | 2131.7 | Semi standard non polar | 33892256 | | N-Formyl-L-glutamic acid,2TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@@H](C(=O)O)N(C=O)[Si](C)(C)C(C)(C)C | 2212.3 | Semi standard non polar | 33892256 | | N-Formyl-L-glutamic acid,2TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC(=O)[C@H](CCC(=O)O)N(C=O)[Si](C)(C)C(C)(C)C | 2192.8 | Semi standard non polar | 33892256 | | N-Formyl-L-glutamic acid,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@@H](C(=O)O[Si](C)(C)C(C)(C)C)N(C=O)[Si](C)(C)C(C)(C)C | 2406.2 | Semi standard non polar | 33892256 | | N-Formyl-L-glutamic acid,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@@H](C(=O)O[Si](C)(C)C(C)(C)C)N(C=O)[Si](C)(C)C(C)(C)C | 2386.1 | Standard non polar | 33892256 | | N-Formyl-L-glutamic acid,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)CC[C@@H](C(=O)O[Si](C)(C)C(C)(C)C)N(C=O)[Si](C)(C)C(C)(C)C | 2367.4 | Standard polar | 33892256 |
|
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - N-Formyl-L-glutamic acid GC-MS (Non-derivatized) - 70eV, Positive | splash10-055f-9500000000-87e21ed84f3e07a0525e | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - N-Formyl-L-glutamic acid GC-MS (2 TMS) - 70eV, Positive | splash10-0udi-5090000000-9bdfed547fb095a666a7 | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - N-Formyl-L-glutamic acid GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - N-Formyl-L-glutamic acid 10V, Positive-QTOF | splash10-0a59-0900000000-56d7b5efa61129bb9f2a | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - N-Formyl-L-glutamic acid 20V, Positive-QTOF | splash10-0f89-1900000000-f0cf3aae7ee3fc5cdc38 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - N-Formyl-L-glutamic acid 40V, Positive-QTOF | splash10-0ue9-9700000000-81fd656d12663befc16e | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - N-Formyl-L-glutamic acid 10V, Negative-QTOF | splash10-05fs-0900000000-e09e113f64d6b1d6f838 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - N-Formyl-L-glutamic acid 20V, Negative-QTOF | splash10-0fba-1900000000-df4e50eec3cd34d33f16 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - N-Formyl-L-glutamic acid 40V, Negative-QTOF | splash10-002f-9300000000-0e7670e199ce4fc65a9a | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - N-Formyl-L-glutamic acid 10V, Positive-QTOF | splash10-053r-0900000000-6fe927d19787d8c02604 | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - N-Formyl-L-glutamic acid 20V, Positive-QTOF | splash10-0f8i-5900000000-f41841dd571e9833fae3 | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - N-Formyl-L-glutamic acid 40V, Positive-QTOF | splash10-0084-9000000000-97a1a533c93f062e0524 | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - N-Formyl-L-glutamic acid 10V, Negative-QTOF | splash10-00b9-0900000000-db871cdf7055dba8e199 | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - N-Formyl-L-glutamic acid 20V, Negative-QTOF | splash10-0f6x-6900000000-421ff60b4e2fe0df43f0 | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - N-Formyl-L-glutamic acid 40V, Negative-QTOF | splash10-0006-9000000000-5a41b69317afca4bfee9 | 2021-09-24 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum |
IR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M-H]-) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+H]+) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+Na]+) | 2023-02-03 | FELIX lab | View Spectrum |
|
|---|