| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected but not Quantified |
|---|
| Creation Date | 2006-08-13 10:23:06 UTC |
|---|
| Update Date | 2023-02-21 17:16:54 UTC |
|---|
| HMDB ID | HMDB0004077 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 4,6-Dihydroxyquinoline |
|---|
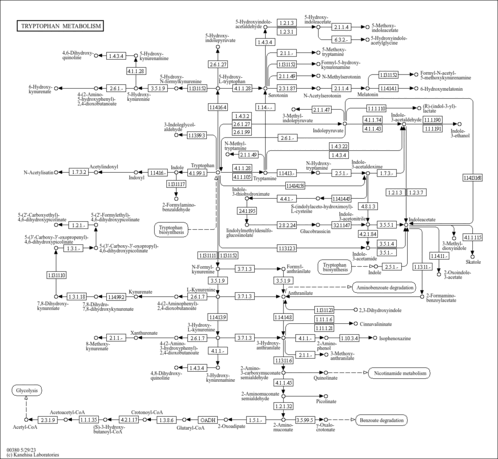
| Description | 4,6-Dihydroxyquinoline, also known as quinoline-4,6-diol, belongs to the class of organic compounds known as hydroxyquinolones. Hydroxyquinolones are compounds containing a quinoline moiety bearing a hydroxyl group and a ketone. Quinoline or benzo[b]pyridine is a bicyclic compound that consists of benzene fused to a pyridine. 4,6-Dihydroxyquinoline exists in all living organisms, ranging from bacteria to humans. In humans, 4,6-dihydroxyquinoline is involved in the tryptophan metabolism pathway. 4,6-Dihydroxyquinoline has been detected, but not quantified in, a few different foods, such as anatidaes (Anatidae), chickens (Gallus gallus), and domestic pigs (Sus scrofa domestica). This could make 4,6-dihydroxyquinoline a potential biomarker for the consumption of these foods. 4,6-Dihydroxyquinoline is a primary metabolite. Primary metabolites are metabolically or physiologically essential metabolites. They are directly involved in an organism’s growth, development or reproduction. Based on a literature review a significant number of articles have been published on 4,6-Dihydroxyquinoline. |
|---|
| Structure | InChI=1S/C9H7NO2/c11-6-1-2-8-7(5-6)9(12)3-4-10-8/h1-5,11H,(H,10,12) |
|---|
| Synonyms | | Value | Source |
|---|
| 6-Hydroxy-1H-quinolin-4-one | ChEBI | | Quinoline-4,6-diol | Kegg | | 4,6-Quinolinediol | HMDB |
|
|---|
| Chemical Formula | C9H7NO2 |
|---|
| Average Molecular Weight | 161.1574 |
|---|
| Monoisotopic Molecular Weight | 161.047678473 |
|---|
| IUPAC Name | quinoline-4,6-diol |
|---|
| Traditional Name | 4,6-dihydroxyquinoline |
|---|
| CAS Registry Number | 3517-61-1 |
|---|
| SMILES | OC1=CC2=C(O)C=CN=C2C=C1 |
|---|
| InChI Identifier | InChI=1S/C9H7NO2/c11-6-1-2-8-7(5-6)9(12)3-4-10-8/h1-5,11H,(H,10,12) |
|---|
| InChI Key | XFALURCRIGINGT-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as hydroxyquinolones. Hydroxyquinolones are compounds containing a quinoline moiety bearing a hydroxyl group and a ketone. Quinoline or benzo[b]pyridine is a bicyclic compound that consists of benzene fused to a pyridine. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Quinolines and derivatives |
|---|
| Sub Class | Quinolones and derivatives |
|---|
| Direct Parent | Hydroxyquinolones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Hydroxyquinolone
- Dihydroquinolone
- Hydroxyquinoline
- Dihydroquinoline
- 1-hydroxy-2-unsubstituted benzenoid
- Pyridine
- Benzenoid
- Heteroaromatic compound
- Vinylogous amide
- Azacycle
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Not Available |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| 4,6-Dihydroxyquinoline,1TMS,isomer #1 | C[Si](C)(C)OC1=CC=C2N=CC=C(O)C2=C1 | 1869.4 | Semi standard non polar | 33892256 | | 4,6-Dihydroxyquinoline,1TMS,isomer #2 | C[Si](C)(C)OC1=CC=NC2=CC=C(O)C=C12 | 1843.5 | Semi standard non polar | 33892256 | | 4,6-Dihydroxyquinoline,2TMS,isomer #1 | C[Si](C)(C)OC1=CC=C2N=CC=C(O[Si](C)(C)C)C2=C1 | 1884.3 | Semi standard non polar | 33892256 | | 4,6-Dihydroxyquinoline,1TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC1=CC=C2N=CC=C(O)C2=C1 | 2121.8 | Semi standard non polar | 33892256 | | 4,6-Dihydroxyquinoline,1TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OC1=CC=NC2=CC=C(O)C=C12 | 2115.3 | Semi standard non polar | 33892256 | | 4,6-Dihydroxyquinoline,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC1=CC=C2N=CC=C(O[Si](C)(C)C(C)(C)C)C2=C1 | 2417.1 | Semi standard non polar | 33892256 |
|
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - 4,6-Dihydroxyquinoline GC-MS (Non-derivatized) - 70eV, Positive | splash10-01q9-0900000000-5a26ca29602b82b947ec | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 4,6-Dihydroxyquinoline GC-MS (2 TMS) - 70eV, Positive | splash10-00ec-4190000000-4bb8d953c5850dd96f48 | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 4,6-Dihydroxyquinoline GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 4,6-Dihydroxyquinoline 10V, Positive-QTOF | splash10-03di-0900000000-2b4e8673d9953857188d | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 4,6-Dihydroxyquinoline 20V, Positive-QTOF | splash10-03di-0900000000-664352c4cf4241c0a1c4 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 4,6-Dihydroxyquinoline 40V, Positive-QTOF | splash10-053r-2900000000-05e37aff72635356f2cc | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 4,6-Dihydroxyquinoline 10V, Negative-QTOF | splash10-03di-0900000000-34a29a4deafcb0ad7c33 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 4,6-Dihydroxyquinoline 20V, Negative-QTOF | splash10-03di-0900000000-06fb975c74de0f90f723 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 4,6-Dihydroxyquinoline 40V, Negative-QTOF | splash10-053r-0900000000-39deb8142073ba5b68ae | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 4,6-Dihydroxyquinoline 10V, Positive-QTOF | splash10-03di-0900000000-d2b094746088a17847e1 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 4,6-Dihydroxyquinoline 20V, Positive-QTOF | splash10-03di-0900000000-d9bf4aaec04c4b90185a | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 4,6-Dihydroxyquinoline 40V, Positive-QTOF | splash10-0159-4900000000-d7445a5c12b24666d75a | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 4,6-Dihydroxyquinoline 10V, Negative-QTOF | splash10-03di-0900000000-29ea448bd0cee5fb98df | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 4,6-Dihydroxyquinoline 20V, Negative-QTOF | splash10-03di-0900000000-02e9863a91ed972b84eb | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 4,6-Dihydroxyquinoline 40V, Negative-QTOF | splash10-053r-5900000000-f7f1ae1acee5ecfdcc35 | 2021-09-22 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum |
IR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M-H]-) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+H]+) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+Na]+) | 2023-02-03 | FELIX lab | View Spectrum |
|
|---|