| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Expected but not Quantified |
|---|
| Creation Date | 2006-08-13 17:42:43 UTC |
|---|
| Update Date | 2023-02-21 17:17:01 UTC |
|---|
| HMDB ID | HMDB0004369 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | N-Methylserotonin |
|---|
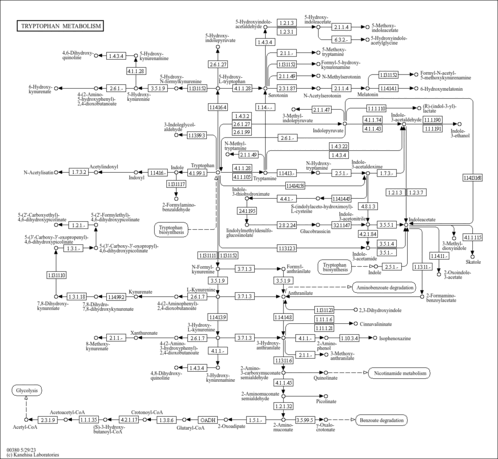
| Description | N-Methylserotonin, also known as lopac-m-1514, belongs to the class of organic compounds known as serotonins. Serotonins are compounds containing a serotonin moiety, which consists of an indole that bears an aminoethyl a position 2 and a hydroxyl group at position 5. Chemically, it is a derivative of serotonin in which a methyl group resides at its alkyl amine. N-Methylserotonin is a very strong basic compound (based on its pKa). N-methylserotonin and S-adenosylhomocysteine can be biosynthesized from serotonin and S-adenosylmethionine through the action of the enzyme indolethylamine N-methyltransferase. These include the plants, Actaea racemosa (black cohosh) and Zanthoxylum piperitum, the Green and Golden Bell Frog, Litoria aurea, and Amanita mushrooms. The compound binds to several serotonin receptors, including the 5-HT7 and 5-HT1A receptors, with high affinity (IC50 ≤ 2 nM) and selectivity, and displays agonist activity; besides its direct interaction with the serotonin receptors, N-methylserotonin also acts as a selective serotonin reuptake inhibitor. N-Methylserotonin is not scheduled at the federal level in the United States, but could be considered an analog (of bufotenin), in which case, sales or possession intended for human consumption could be prosecuted under the Federal Analog Act. In humans, N-methylserotonin is involved in tryptophan metabolism. Outside of the human body, N-Methylserotonin has been detected, but not quantified in, citrus. This could make N-methylserotonin a potential biomarker for the consumption of these foods. N-Methylserotonin is a tryptamine alkaloid. Florida N-Methylserotonin is a Schedule I controlled substance in the state of Florida making it illegal to buy, sell, or possess in Florida. It is also called Nω-methylserotonin (Nω-methyl-5-hydroxytryptamine) to distinguish it from tryptamine-derived compounds in which a methyl group is bonded to the nitrogen atom of the indole group. |
|---|
| Structure | InChI=1S/C11H14N2O/c1-12-5-4-8-7-13-11-3-2-9(14)6-10(8)11/h2-3,6-7,12-14H,4-5H2,1H3 |
|---|
| Synonyms | | Value | Source |
|---|
| N-Methylserotonin oxalate salt | HMDB | | 3-(2-(Methylamino)ethyl)-1H-indol-5-ol | HMDB | | 3-[2-(Methylamino)ethyl]-1H-indol-5-ol | HMDB | | Lopac-m-1514 | HMDB |
|
|---|
| Chemical Formula | C11H14N2O |
|---|
| Average Molecular Weight | 190.2417 |
|---|
| Monoisotopic Molecular Weight | 190.11061308 |
|---|
| IUPAC Name | 3-[2-(methylamino)ethyl]-1H-indol-5-ol |
|---|
| Traditional Name | N-methylserotonin |
|---|
| CAS Registry Number | 1134-01-6 |
|---|
| SMILES | CNCCC1=CNC2=C1C=C(O)C=C2 |
|---|
| InChI Identifier | InChI=1S/C11H14N2O/c1-12-5-4-8-7-13-11-3-2-9(14)6-10(8)11/h2-3,6-7,12-14H,4-5H2,1H3 |
|---|
| InChI Key | ASUSBMNYRHGZIG-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as serotonins. Serotonins are compounds containing a serotonin moiety, which consists of an indole that bears an aminoethyl a position 2 and a hydroxyl group at position 5. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Indoles and derivatives |
|---|
| Sub Class | Tryptamines and derivatives |
|---|
| Direct Parent | Serotonins |
|---|
| Alternative Parents | |
|---|
| Substituents | - Serotonin
- Hydroxyindole
- 3-alkylindole
- Indole
- 1-hydroxy-2-unsubstituted benzenoid
- Aralkylamine
- Benzenoid
- Substituted pyrrole
- Pyrrole
- Heteroaromatic compound
- Secondary aliphatic amine
- Secondary amine
- Azacycle
- Amine
- Hydrocarbon derivative
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Not Available |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| N-Methylserotonin,1TMS,isomer #1 | CNCCC1=C[NH]C2=CC=C(O[Si](C)(C)C)C=C12 | 1986.2 | Semi standard non polar | 33892256 | | N-Methylserotonin,1TMS,isomer #2 | CN(CCC1=C[NH]C2=CC=C(O)C=C12)[Si](C)(C)C | 2215.7 | Semi standard non polar | 33892256 | | N-Methylserotonin,1TMS,isomer #3 | CNCCC1=CN([Si](C)(C)C)C2=CC=C(O)C=C12 | 2056.9 | Semi standard non polar | 33892256 | | N-Methylserotonin,2TMS,isomer #1 | CN(CCC1=C[NH]C2=CC=C(O[Si](C)(C)C)C=C12)[Si](C)(C)C | 2220.1 | Semi standard non polar | 33892256 | | N-Methylserotonin,2TMS,isomer #1 | CN(CCC1=C[NH]C2=CC=C(O[Si](C)(C)C)C=C12)[Si](C)(C)C | 2285.8 | Standard non polar | 33892256 | | N-Methylserotonin,2TMS,isomer #1 | CN(CCC1=C[NH]C2=CC=C(O[Si](C)(C)C)C=C12)[Si](C)(C)C | 2362.3 | Standard polar | 33892256 | | N-Methylserotonin,2TMS,isomer #2 | CNCCC1=CN([Si](C)(C)C)C2=CC=C(O[Si](C)(C)C)C=C12 | 2041.7 | Semi standard non polar | 33892256 | | N-Methylserotonin,2TMS,isomer #2 | CNCCC1=CN([Si](C)(C)C)C2=CC=C(O[Si](C)(C)C)C=C12 | 2139.9 | Standard non polar | 33892256 | | N-Methylserotonin,2TMS,isomer #2 | CNCCC1=CN([Si](C)(C)C)C2=CC=C(O[Si](C)(C)C)C=C12 | 2265.6 | Standard polar | 33892256 | | N-Methylserotonin,2TMS,isomer #3 | CN(CCC1=CN([Si](C)(C)C)C2=CC=C(O)C=C12)[Si](C)(C)C | 2296.0 | Semi standard non polar | 33892256 | | N-Methylserotonin,2TMS,isomer #3 | CN(CCC1=CN([Si](C)(C)C)C2=CC=C(O)C=C12)[Si](C)(C)C | 2368.1 | Standard non polar | 33892256 | | N-Methylserotonin,2TMS,isomer #3 | CN(CCC1=CN([Si](C)(C)C)C2=CC=C(O)C=C12)[Si](C)(C)C | 2391.5 | Standard polar | 33892256 | | N-Methylserotonin,3TMS,isomer #1 | CN(CCC1=CN([Si](C)(C)C)C2=CC=C(O[Si](C)(C)C)C=C12)[Si](C)(C)C | 2293.6 | Semi standard non polar | 33892256 | | N-Methylserotonin,3TMS,isomer #1 | CN(CCC1=CN([Si](C)(C)C)C2=CC=C(O[Si](C)(C)C)C=C12)[Si](C)(C)C | 2305.9 | Standard non polar | 33892256 | | N-Methylserotonin,3TMS,isomer #1 | CN(CCC1=CN([Si](C)(C)C)C2=CC=C(O[Si](C)(C)C)C=C12)[Si](C)(C)C | 2246.5 | Standard polar | 33892256 | | N-Methylserotonin,1TBDMS,isomer #1 | CNCCC1=C[NH]C2=CC=C(O[Si](C)(C)C(C)(C)C)C=C12 | 2252.0 | Semi standard non polar | 33892256 | | N-Methylserotonin,1TBDMS,isomer #2 | CN(CCC1=C[NH]C2=CC=C(O)C=C12)[Si](C)(C)C(C)(C)C | 2462.8 | Semi standard non polar | 33892256 | | N-Methylserotonin,1TBDMS,isomer #3 | CNCCC1=CN([Si](C)(C)C(C)(C)C)C2=CC=C(O)C=C12 | 2305.2 | Semi standard non polar | 33892256 | | N-Methylserotonin,2TBDMS,isomer #1 | CN(CCC1=C[NH]C2=CC=C(O[Si](C)(C)C(C)(C)C)C=C12)[Si](C)(C)C(C)(C)C | 2710.0 | Semi standard non polar | 33892256 | | N-Methylserotonin,2TBDMS,isomer #1 | CN(CCC1=C[NH]C2=CC=C(O[Si](C)(C)C(C)(C)C)C=C12)[Si](C)(C)C(C)(C)C | 2733.2 | Standard non polar | 33892256 | | N-Methylserotonin,2TBDMS,isomer #1 | CN(CCC1=C[NH]C2=CC=C(O[Si](C)(C)C(C)(C)C)C=C12)[Si](C)(C)C(C)(C)C | 2585.5 | Standard polar | 33892256 | | N-Methylserotonin,2TBDMS,isomer #2 | CNCCC1=CN([Si](C)(C)C(C)(C)C)C2=CC=C(O[Si](C)(C)C(C)(C)C)C=C12 | 2504.2 | Semi standard non polar | 33892256 | | N-Methylserotonin,2TBDMS,isomer #2 | CNCCC1=CN([Si](C)(C)C(C)(C)C)C2=CC=C(O[Si](C)(C)C(C)(C)C)C=C12 | 2583.3 | Standard non polar | 33892256 | | N-Methylserotonin,2TBDMS,isomer #2 | CNCCC1=CN([Si](C)(C)C(C)(C)C)C2=CC=C(O[Si](C)(C)C(C)(C)C)C=C12 | 2468.6 | Standard polar | 33892256 | | N-Methylserotonin,2TBDMS,isomer #3 | CN(CCC1=CN([Si](C)(C)C(C)(C)C)C2=CC=C(O)C=C12)[Si](C)(C)C(C)(C)C | 2723.1 | Semi standard non polar | 33892256 | | N-Methylserotonin,2TBDMS,isomer #3 | CN(CCC1=CN([Si](C)(C)C(C)(C)C)C2=CC=C(O)C=C12)[Si](C)(C)C(C)(C)C | 2792.8 | Standard non polar | 33892256 | | N-Methylserotonin,2TBDMS,isomer #3 | CN(CCC1=CN([Si](C)(C)C(C)(C)C)C2=CC=C(O)C=C12)[Si](C)(C)C(C)(C)C | 2572.4 | Standard polar | 33892256 | | N-Methylserotonin,3TBDMS,isomer #1 | CN(CCC1=CN([Si](C)(C)C(C)(C)C)C2=CC=C(O[Si](C)(C)C(C)(C)C)C=C12)[Si](C)(C)C(C)(C)C | 2953.0 | Semi standard non polar | 33892256 | | N-Methylserotonin,3TBDMS,isomer #1 | CN(CCC1=CN([Si](C)(C)C(C)(C)C)C2=CC=C(O[Si](C)(C)C(C)(C)C)C=C12)[Si](C)(C)C(C)(C)C | 2930.1 | Standard non polar | 33892256 | | N-Methylserotonin,3TBDMS,isomer #1 | CN(CCC1=CN([Si](C)(C)C(C)(C)C)C2=CC=C(O[Si](C)(C)C(C)(C)C)C=C12)[Si](C)(C)C(C)(C)C | 2572.0 | Standard polar | 33892256 |
|
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - N-Methylserotonin GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9300000000-514cf662a4e6c5a603df | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - N-Methylserotonin GC-MS (1 TMS) - 70eV, Positive | splash10-0006-9120000000-4c9438c3246e1f92d1f5 | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - N-Methylserotonin GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - N-Methylserotonin 10V, Positive-QTOF | splash10-01ox-0900000000-646686588892f9e597f5 | 2015-04-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - N-Methylserotonin 20V, Positive-QTOF | splash10-03dl-1900000000-a768b9399c8fcac6cd0e | 2015-04-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - N-Methylserotonin 40V, Positive-QTOF | splash10-01q9-2900000000-543fe5365e1fa682b60e | 2015-04-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - N-Methylserotonin 10V, Negative-QTOF | splash10-000i-0900000000-b4a2e8f1bf6e3e782690 | 2015-04-25 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - N-Methylserotonin 20V, Negative-QTOF | splash10-000i-0900000000-970029b3b4e6dd32e53a | 2015-04-25 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - N-Methylserotonin 40V, Negative-QTOF | splash10-001i-2900000000-9851a5693e2c284d3b9d | 2015-04-25 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - N-Methylserotonin 10V, Positive-QTOF | splash10-01ox-0900000000-068fac34efa1b5ba779c | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - N-Methylserotonin 20V, Positive-QTOF | splash10-03di-0900000000-46a702a381d3892e40b4 | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - N-Methylserotonin 40V, Positive-QTOF | splash10-0f8c-2900000000-d17d6394f323b90f889c | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - N-Methylserotonin 10V, Negative-QTOF | splash10-000i-0900000000-737a601f9d97628ca508 | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - N-Methylserotonin 20V, Negative-QTOF | splash10-0019-0900000000-58467315e6d4795f2488 | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - N-Methylserotonin 40V, Negative-QTOF | splash10-001i-2900000000-cdd5d680769ece6297e4 | 2021-09-24 | Wishart Lab | View Spectrum |
IR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M-H]-) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+H]+) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+Na]+) | 2023-02-03 | FELIX lab | View Spectrum |
|
|---|
| Synthesis Reference | Somei, Masanori; Yamada, Fumio; Kurauchi, Takashi; Nagahama, Yoshiyuki; Hasegawa, Masakazu; Yamada, Koji; Teranishi, Sakiko; Sato, Haruhiko; Kaneko, Chikara. The chemistry of indoles. CIII. Simple syntheses of serotonin, N-methylserotonin, bufotenine, 5-m |
|---|