| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Expected but not Quantified |
|---|
| Creation Date | 2007-05-22 17:50:52 UTC |
|---|
| Update Date | 2022-03-07 02:49:30 UTC |
|---|
| HMDB ID | HMDB0006217 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 9-cis-Retinol |
|---|
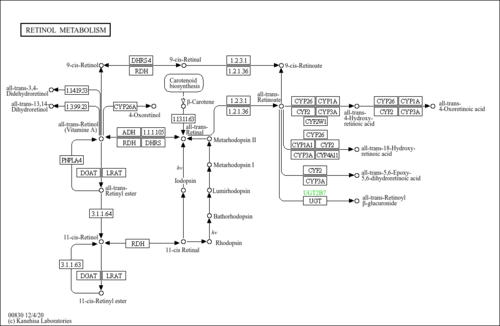
| Description | 9-cis-retinol is a retinoid. Retinoids (vitamin A and its analogs) are essential dietary substances that are needed by mammals for reproduction, normal embryogenesis, growth, vision, and maintaining normal cellular differentiation and the integrity of the immune system. Within cells, retinoids regulate gene transcription acting through ligand-dependent transcription factors, the retinoic acid receptors (RARs), and the retinoid X receptors (RXRs). All-trans-retinoic acid binds only to RARs with high affinity, whereas its 9-cis isomer binds with high affinity to both RARs and RXRs. The actions of all-trans- and 9-cis-retinoic acid in regulating cellular responses are distinct and not interchangeable. (PMID: 9115228 ). |
|---|
| Structure | C\C(=C/CO)\C=C\C=C(\C)/C=C/C1=C(C)CCCC1(C)C InChI=1S/C20H30O/c1-16(8-6-9-17(2)13-15-21)11-12-19-18(3)10-7-14-20(19,4)5/h6,8-9,11-13,21H,7,10,14-15H2,1-5H3/b9-6+,12-11+,16-8-,17-13+ |
|---|
| Synonyms | | Value | Source |
|---|
| (2E,4E,6Z,8E)-3,7-Dimethyl-9-(2,6,6-trimethylcyclohex-1-en-1-yl)nona-2,4,6,8-tetraen-1-ol | ChEBI | | (9cis)-Retinol | ChEBI | | 9-cis Retinol | HMDB |
|
|---|
| Chemical Formula | C20H30O |
|---|
| Average Molecular Weight | 286.4516 |
|---|
| Monoisotopic Molecular Weight | 286.229665582 |
|---|
| IUPAC Name | (2E,4E,6Z,8E)-3,7-dimethyl-9-(2,6,6-trimethylcyclohex-1-en-1-yl)nona-2,4,6,8-tetraen-1-ol |
|---|
| Traditional Name | 9-cis-retinol |
|---|
| CAS Registry Number | 22737-97-9 |
|---|
| SMILES | C\C(=C/CO)\C=C\C=C(\C)/C=C/C1=C(C)CCCC1(C)C |
|---|
| InChI Identifier | InChI=1S/C20H30O/c1-16(8-6-9-17(2)13-15-21)11-12-19-18(3)10-7-14-20(19,4)5/h6,8-9,11-13,21H,7,10,14-15H2,1-5H3/b9-6+,12-11+,16-8-,17-13+ |
|---|
| InChI Key | FPIPGXGPPPQFEQ-MKOSUFFBSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as retinoids. These are oxygenated derivatives of 3,7-dimethyl-1-(2,6,6-trimethylcyclohex-1-enyl)nona-1,3,5,7-tetraene and derivatives thereof. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Retinoids |
|---|
| Direct Parent | Retinoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Retinoid skeleton
- Diterpenoid
- Fatty alcohol
- Fatty acyl
- Organic oxygen compound
- Hydrocarbon derivative
- Primary alcohol
- Organooxygen compound
- Alcohol
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Not Available |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Kovats Retention IndicesUnderivatizedDerivatized |
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - 9-cis-Retinol GC-MS (Non-derivatized) - 70eV, Positive | splash10-00di-1190000000-346a3e44c44d46353e80 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 9-cis-Retinol GC-MS (1 TMS) - 70eV, Positive | splash10-0006-7139000000-7242603c3f7f12c824cd | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 9-cis-Retinol GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 9-cis-Retinol 10V, Positive-QTOF | splash10-00kr-0490000000-97ed49d705440e4d32e7 | 2016-06-03 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 9-cis-Retinol 20V, Positive-QTOF | splash10-00ks-3930000000-7c5bfbd8b838b97dc2aa | 2016-06-03 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 9-cis-Retinol 40V, Positive-QTOF | splash10-0fri-9820000000-6a49bab0c891ffe1e5b2 | 2016-06-03 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 9-cis-Retinol 10V, Negative-QTOF | splash10-000i-0090000000-d0ff1cdc72141d0daa0e | 2016-08-03 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 9-cis-Retinol 20V, Negative-QTOF | splash10-0a4r-0090000000-c0a067ff8ed271b80945 | 2016-08-03 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 9-cis-Retinol 40V, Negative-QTOF | splash10-00ku-4590000000-cfc35aba420e707075f7 | 2016-08-03 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 9-cis-Retinol 10V, Negative-QTOF | splash10-0a4i-0090000000-fe93459d3069e264b77f | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 9-cis-Retinol 20V, Negative-QTOF | splash10-05mx-0190000000-f81760535f6050971f92 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 9-cis-Retinol 40V, Negative-QTOF | splash10-00li-0960000000-4dbc516b202b90a6c265 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 9-cis-Retinol 10V, Positive-QTOF | splash10-015i-1890000000-fb68e645ac6c84dc722c | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 9-cis-Retinol 20V, Positive-QTOF | splash10-05a9-2910000000-aa07b89a9cfe132b8725 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 9-cis-Retinol 40V, Positive-QTOF | splash10-06dl-5900000000-6de72efc591b252620c9 | 2021-09-22 | Wishart Lab | View Spectrum |
|
|---|