| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Expected but not Quantified |
|---|
| Creation Date | 2007-05-23 09:54:11 UTC |
|---|
| Update Date | 2021-09-14 15:00:11 UTC |
|---|
| HMDB ID | HMDB0006458 |
|---|
| Secondary Accession Numbers | - HMDB0006949
- HMDB06458
- HMDB06949
|
|---|
| Metabolite Identification |
|---|
| Common Name | D-Lactaldehyde |
|---|
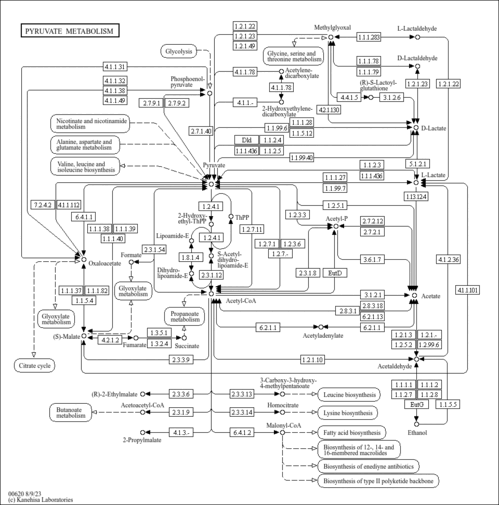
| Description | D-Lactaldehyde belongs to the class of organic compounds known as alpha-hydroxyaldehydes. These are organic compounds containing an aldehyde substituted with a hydroxyl group on the adjacent carbon. D-Lactaldehyde exists in all living species, ranging from bacteria to plants to humans. In humans, D-lactaldehyde is involved in the metabolic disorder called the leigh syndrome pathway. D-Lactaldehyde has been detected, but not quantified in, a few different foods, such as anatidaes (Anatidae), chickens (Gallus gallus), and domestic pigs (Sus scrofa domestica). This could make D-lactaldehyde a potential biomarker for the consumption of these foods. D-Lactaldehyde is a primary metabolite. Primary metabolites are metabolically or physiologically essential metabolites. They are directly involved in an organism’s growth, development or reproduction. Based on a literature review a small amount of articles have been published on D-Lactaldehyde. |
|---|
| Structure | InChI=1S/C3H6O2/c1-3(5)2-4/h2-3,5H,1H3/t3-/m1/s1 |
|---|
| Synonyms | | Value | Source |
|---|
| D-2-Hydroxypropionaldehyde | ChEBI | | (2R)-2-Hydroxypropanal | HMDB | | (R)-Lactaldehyde | HMDB |
|
|---|
| Chemical Formula | C3H6O2 |
|---|
| Average Molecular Weight | 74.0785 |
|---|
| Monoisotopic Molecular Weight | 74.036779436 |
|---|
| IUPAC Name | (2R)-2-hydroxypropanal |
|---|
| Traditional Name | D-lactaldehyde |
|---|
| CAS Registry Number | 3946-09-6 |
|---|
| SMILES | C[C@@H](O)C=O |
|---|
| InChI Identifier | InChI=1S/C3H6O2/c1-3(5)2-4/h2-3,5H,1H3/t3-/m1/s1 |
|---|
| InChI Key | BSABBBMNWQWLLU-GSVOUGTGSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as alpha-hydroxyaldehydes. These are organic compounds containing an aldehyde substituted with a hydroxyl group on the adjacent carbon. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbonyl compounds |
|---|
| Direct Parent | Alpha-hydroxyaldehydes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-hydroxyaldehyde
- Secondary alcohol
- Organic oxide
- Hydrocarbon derivative
- Short-chain aldehyde
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Not Available |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Retention Times Underivatized| Chromatographic Method | Retention Time | Reference |
|---|
| Measured using a Waters Acquity ultraperformance liquid chromatography (UPLC) ethylene-bridged hybrid (BEH) C18 column (100 mm × 2.1 mm; 1.7 μmparticle diameter). Predicted by Afia on May 17, 2022. Predicted by Afia on May 17, 2022. | 1.0 minutes | 32390414 | | Predicted by Siyang on May 30, 2022 | 8.9297 minutes | 33406817 | | Predicted by Siyang using ReTip algorithm on June 8, 2022 | 2.34 minutes | 32390414 | | AjsUoB = Accucore 150 Amide HILIC with 10mM Ammonium Formate, 0.1% Formic Acid | 162.4 seconds | 40023050 | | Fem_Long = Waters ACQUITY UPLC HSS T3 C18 with Water:MeOH and 0.1% Formic Acid | 858.5 seconds | 40023050 | | Fem_Lipids = Ascentis Express C18 with (60:40 water:ACN):(90:10 IPA:ACN) and 10mM NH4COOH + 0.1% Formic Acid | 359.5 seconds | 40023050 | | Life_Old = Waters ACQUITY UPLC BEH C18 with Water:(20:80 acetone:ACN) and 0.1% Formic Acid | 95.7 seconds | 40023050 | | Life_New = RP Waters ACQUITY UPLC HSS T3 C18 with Water:(30:70 MeOH:ACN) and 0.1% Formic Acid | 251.2 seconds | 40023050 | | RIKEN = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 67.9 seconds | 40023050 | | Eawag_XBridgeC18 = XBridge C18 3.5u 2.1x50 mm with Water:MeOH and 0.1% Formic Acid | 269.4 seconds | 40023050 | | BfG_NTS_RP1 =Agilent Zorbax Eclipse Plus C18 (2.1 mm x 150 mm, 3.5 um) with Water:ACN and 0.1% Formic Acid | 334.3 seconds | 40023050 | | HILIC_BDD_2 = Merck SeQuant ZIC-HILIC with ACN(0.1% formic acid):water(16 mM ammonium formate) | 200.2 seconds | 40023050 | | UniToyama_Atlantis = RP Waters Atlantis T3 (2.1 x 150 mm, 5 um) with ACN:Water and 0.1% Formic Acid | 606.5 seconds | 40023050 | | BDD_C18 = Hypersil Gold 1.9µm C18 with Water:ACN and 0.1% Formic Acid | 129.5 seconds | 40023050 | | UFZ_Phenomenex = Kinetex Core-Shell C18 2.6 um, 3.0 x 100 mm, Phenomenex with Water:MeOH and 0.1% Formic Acid | 825.3 seconds | 40023050 | | SNU_RIKEN_POS = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 230.1 seconds | 40023050 | | RPMMFDA = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 279.5 seconds | 40023050 | | MTBLS87 = Merck SeQuant ZIC-pHILIC column with ACN:Water and :ammonium carbonate | 576.3 seconds | 40023050 | | KI_GIAR_zic_HILIC_pH2_7 = Merck SeQuant ZIC-HILIC with ACN:Water and 0.1% FA | 298.7 seconds | 40023050 | | Meister zic-pHILIC pH9.3 = Merck SeQuant ZIC-pHILIC column with ACN:Water 5mM NH4Ac pH9.3 and 5mM ammonium acetate in water | 231.2 seconds | 40023050 |
Predicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| D-Lactaldehyde,1TMS,isomer #1 | C[C@H](C=O)O[Si](C)(C)C | 829.4 | Semi standard non polar | 33892256 | | D-Lactaldehyde,1TMS,isomer #2 | CC(O)=CO[Si](C)(C)C | 926.3 | Semi standard non polar | 33892256 | | D-Lactaldehyde,2TMS,isomer #1 | CC(=CO[Si](C)(C)C)O[Si](C)(C)C | 1052.3 | Semi standard non polar | 33892256 | | D-Lactaldehyde,2TMS,isomer #1 | CC(=CO[Si](C)(C)C)O[Si](C)(C)C | 986.6 | Standard non polar | 33892256 | | D-Lactaldehyde,2TMS,isomer #1 | CC(=CO[Si](C)(C)C)O[Si](C)(C)C | 1016.7 | Standard polar | 33892256 | | D-Lactaldehyde,1TBDMS,isomer #1 | C[C@H](C=O)O[Si](C)(C)C(C)(C)C | 1062.2 | Semi standard non polar | 33892256 | | D-Lactaldehyde,1TBDMS,isomer #2 | CC(O)=CO[Si](C)(C)C(C)(C)C | 1149.3 | Semi standard non polar | 33892256 | | D-Lactaldehyde,2TBDMS,isomer #1 | CC(=CO[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 1469.6 | Semi standard non polar | 33892256 | | D-Lactaldehyde,2TBDMS,isomer #1 | CC(=CO[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 1442.1 | Standard non polar | 33892256 | | D-Lactaldehyde,2TBDMS,isomer #1 | CC(=CO[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 1345.9 | Standard polar | 33892256 |
|
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - D-Lactaldehyde GC-MS (Non-derivatized) - 70eV, Positive | splash10-056v-9000000000-fe4416498788a968e467 | 2016-09-22 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - D-Lactaldehyde GC-MS (1 TMS) - 70eV, Positive | splash10-00fr-9300000000-12b08b93303d8cbf5c3a | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - D-Lactaldehyde GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - D-Lactaldehyde 10V, Positive-QTOF | splash10-004i-9000000000-1aa2338222aff31e5e0f | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - D-Lactaldehyde 20V, Positive-QTOF | splash10-056r-9000000000-c450e557b2fe7c5dc5a1 | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - D-Lactaldehyde 40V, Positive-QTOF | splash10-0a6r-9000000000-dceca37df952939d1084 | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - D-Lactaldehyde 10V, Negative-QTOF | splash10-00di-9000000000-cd6930d73efb436bc0ff | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - D-Lactaldehyde 20V, Negative-QTOF | splash10-00di-9000000000-97d1b594dd3b1d9b08b8 | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - D-Lactaldehyde 40V, Negative-QTOF | splash10-0a4i-9000000000-90fa8913085480b29a8a | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - D-Lactaldehyde 10V, Positive-QTOF | splash10-0a4i-9000000000-feb30b99fe1a3132abb8 | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - D-Lactaldehyde 20V, Positive-QTOF | splash10-0a4j-9000000000-f5b28df550bdfcf22cc7 | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - D-Lactaldehyde 40V, Positive-QTOF | splash10-052s-9000000000-db6ce3d247485bf9581b | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - D-Lactaldehyde 10V, Negative-QTOF | splash10-05fr-9000000000-518c4b32efb3532122ea | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - D-Lactaldehyde 20V, Negative-QTOF | splash10-05fr-9000000000-90bd5b8f63ecfac6a42a | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - D-Lactaldehyde 40V, Negative-QTOF | splash10-0a4i-9000000000-23a60e5f52ca732e0f56 | 2021-09-24 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum |
|
|---|