| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected and Quantified |
|---|
| Creation Date | 2008-10-16 22:20:23 UTC |
|---|
| Update Date | 2023-02-21 17:17:26 UTC |
|---|
| HMDB ID | HMDB0010738 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 1-Methylxanthine |
|---|
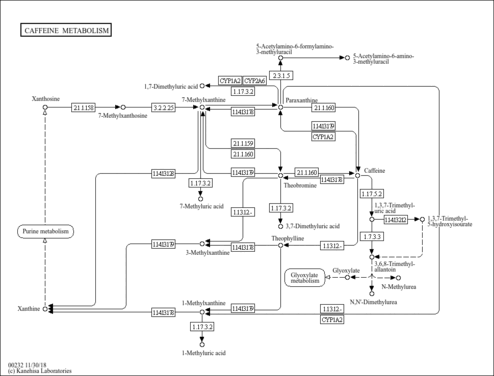
| Description | 1-Methylxanthine belongs to the class of organic compounds known as xanthines. These are purine derivatives with a ketone group conjugated at carbons 2 and 6 of the purine moiety. 1-Methylxanthine exists in all living organisms, ranging from bacteria to humans. In humans, 1-methylxanthine is involved in the caffeine metabolism pathway. 1-Methylxanthine has been detected, but not quantified in, several different foods, such as garden tomatoes (Solanum lycopersicum), lotus (Nelumbo), okras (Abelmoschus esculentus), tamarinds (Tamarindus indica), and chestnuts (Castanea). This could make 1-methylxanthine a potential biomarker for the consumption of these foods. 1-Methylxanthine is a primary metabolite. Primary metabolites are metabolically or physiologically essential metabolites. They are directly involved in an organism’s growth, development or reproduction. Based on a literature review a significant number of articles have been published on 1-Methylxanthine. |
|---|
| Structure | InChI=1S/C6H6N4O2/c1-10-5(11)3-4(8-2-7-3)9-6(10)12/h2H,1H3,(H,7,8)(H,9,12) |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C6H6N4O2 |
|---|
| Average Molecular Weight | 166.1374 |
|---|
| Monoisotopic Molecular Weight | 166.049075456 |
|---|
| IUPAC Name | 2-hydroxy-1-methyl-6,9-dihydro-1H-purin-6-one |
|---|
| Traditional Name | 1-methylxanthine |
|---|
| CAS Registry Number | 6136-37-4 |
|---|
| SMILES | CN1C(=O)NC2=C(NC=N2)C1=O |
|---|
| InChI Identifier | InChI=1S/C6H6N4O2/c1-10-5(11)3-4(8-2-7-3)9-6(10)12/h2H,1H3,(H,7,8)(H,9,12) |
|---|
| InChI Key | MVOYJPOZRLFTCP-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as xanthines. These are purine derivatives with a ketone group conjugated at carbons 2 and 6 of the purine moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Imidazopyrimidines |
|---|
| Sub Class | Purines and purine derivatives |
|---|
| Direct Parent | Xanthines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Xanthine
- 6-oxopurine
- Purinone
- Alkaloid or derivatives
- Pyrimidone
- Pyrimidine
- Azole
- Imidazole
- Heteroaromatic compound
- Vinylogous amide
- Lactam
- Urea
- Azacycle
- Hydrocarbon derivative
- Organic oxide
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Organopnictogen compound
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 5340 mg/L @ 25 °C (est) | The Good Scents Company Information System | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Experimental Collision Cross Sections |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| 1-Methylxanthine,1TMS,isomer #1 | CN1C(=O)C2=C(N=C[NH]2)N([Si](C)(C)C)C1=O | 1887.5 | Semi standard non polar | 33892256 | | 1-Methylxanthine,1TMS,isomer #1 | CN1C(=O)C2=C(N=C[NH]2)N([Si](C)(C)C)C1=O | 2085.5 | Standard non polar | 33892256 | | 1-Methylxanthine,1TMS,isomer #1 | CN1C(=O)C2=C(N=C[NH]2)N([Si](C)(C)C)C1=O | 2822.8 | Standard polar | 33892256 | | 1-Methylxanthine,1TMS,isomer #2 | CN1C(=O)[NH]C2=C(C1=O)N([Si](C)(C)C)C=N2 | 1966.8 | Semi standard non polar | 33892256 | | 1-Methylxanthine,1TMS,isomer #2 | CN1C(=O)[NH]C2=C(C1=O)N([Si](C)(C)C)C=N2 | 2100.3 | Standard non polar | 33892256 | | 1-Methylxanthine,1TMS,isomer #2 | CN1C(=O)[NH]C2=C(C1=O)N([Si](C)(C)C)C=N2 | 2699.8 | Standard polar | 33892256 | | 1-Methylxanthine,2TMS,isomer #1 | CN1C(=O)C2=C(N=CN2[Si](C)(C)C)N([Si](C)(C)C)C1=O | 1951.8 | Semi standard non polar | 33892256 | | 1-Methylxanthine,2TMS,isomer #1 | CN1C(=O)C2=C(N=CN2[Si](C)(C)C)N([Si](C)(C)C)C1=O | 2103.3 | Standard non polar | 33892256 | | 1-Methylxanthine,2TMS,isomer #1 | CN1C(=O)C2=C(N=CN2[Si](C)(C)C)N([Si](C)(C)C)C1=O | 2356.5 | Standard polar | 33892256 | | 1-Methylxanthine,1TBDMS,isomer #1 | CN1C(=O)C2=C(N=C[NH]2)N([Si](C)(C)C(C)(C)C)C1=O | 2089.4 | Semi standard non polar | 33892256 | | 1-Methylxanthine,1TBDMS,isomer #1 | CN1C(=O)C2=C(N=C[NH]2)N([Si](C)(C)C(C)(C)C)C1=O | 2279.6 | Standard non polar | 33892256 | | 1-Methylxanthine,1TBDMS,isomer #1 | CN1C(=O)C2=C(N=C[NH]2)N([Si](C)(C)C(C)(C)C)C1=O | 2791.5 | Standard polar | 33892256 | | 1-Methylxanthine,1TBDMS,isomer #2 | CN1C(=O)[NH]C2=C(C1=O)N([Si](C)(C)C(C)(C)C)C=N2 | 2187.1 | Semi standard non polar | 33892256 | | 1-Methylxanthine,1TBDMS,isomer #2 | CN1C(=O)[NH]C2=C(C1=O)N([Si](C)(C)C(C)(C)C)C=N2 | 2290.0 | Standard non polar | 33892256 | | 1-Methylxanthine,1TBDMS,isomer #2 | CN1C(=O)[NH]C2=C(C1=O)N([Si](C)(C)C(C)(C)C)C=N2 | 2742.7 | Standard polar | 33892256 | | 1-Methylxanthine,2TBDMS,isomer #1 | CN1C(=O)C2=C(N=CN2[Si](C)(C)C(C)(C)C)N([Si](C)(C)C(C)(C)C)C1=O | 2364.8 | Semi standard non polar | 33892256 | | 1-Methylxanthine,2TBDMS,isomer #1 | CN1C(=O)C2=C(N=CN2[Si](C)(C)C(C)(C)C)N([Si](C)(C)C(C)(C)C)C1=O | 2523.3 | Standard non polar | 33892256 | | 1-Methylxanthine,2TBDMS,isomer #1 | CN1C(=O)C2=C(N=CN2[Si](C)(C)C(C)(C)C)N([Si](C)(C)C(C)(C)C)C1=O | 2461.3 | Standard polar | 33892256 |
|
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - 1-Methylxanthine GC-MS (Non-derivatized) - 70eV, Positive | splash10-0apr-3900000000-88bd492326591e730086 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 1-Methylxanthine GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 1-Methylxanthine GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental LC-MS/MS | LC-MS/MS Spectrum - 1-Methylxanthine 40V, Negative-QTOF | splash10-014i-9000000000-6fbdd0235ae20be5daa1 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 1-Methylxanthine 30V, Negative-QTOF | splash10-067i-9400000000-26298794cbed4fbcad6b | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 1-Methylxanthine 20V, Negative-QTOF | splash10-0aor-3900000000-712a7974e530515b891f | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 1-Methylxanthine 10V, Negative-QTOF | splash10-014i-0900000000-69924d9d22c49622a32b | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 1-Methylxanthine NA , positive-QTOF | splash10-014i-0900000000-a729acfc9694f20accb0 | 2020-07-21 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 1-Methylxanthine Orbitrap 3V, positive-QTOF | splash10-014i-0900000000-6d707d0f8b77fb6f3fcd | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 1-Methylxanthine Orbitrap 4V, positive-QTOF | splash10-014i-0900000000-a55efb1f01a8ca4a2d95 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 1-Methylxanthine Orbitrap 4V, positive-QTOF | splash10-02t9-0900000000-858059d2d9ee8414cafd | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 1-Methylxanthine Orbitrap 5V, positive-QTOF | splash10-02t9-0900000000-63a50718538a27464c46 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 1-Methylxanthine Orbitrap 6V, positive-QTOF | splash10-03di-0900000000-a00013c8cdc36103e8a3 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 1-Methylxanthine Orbitrap 8V, positive-QTOF | splash10-03di-2900000000-77efece85a8e1b602d5c | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 1-Methylxanthine Orbitrap 9V, positive-QTOF | splash10-08fr-6900000000-0d9cd06c582f1bf23225 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 1-Methylxanthine Orbitrap 11V, positive-QTOF | splash10-0a4i-9300000000-b73bf7301278872b9e26 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 1-Methylxanthine Orbitrap 14V, positive-QTOF | splash10-0a4i-9100000000-c05924743c475bbab05b | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 1-Methylxanthine Orbitrap 17V, positive-QTOF | splash10-0a4i-9000000000-c01a620479ccd936f5ea | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 1-Methylxanthine n/a 11V, positive-QTOF | splash10-03di-0900000000-9fadde7724e8a2f4bab8 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 1-Methylxanthine n/a 11V, positive-QTOF | splash10-001i-9000000000-5277d916d914013bd114 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 1-Methylxanthine n/a 11V, positive-QTOF | splash10-00di-0900000000-b32aa806de261f5c0e40 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 1-Methylxanthine Orbitrap 2V, positive-QTOF | splash10-03di-1900000000-14f01d07c28c61c92026 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 1-Methylxanthine Orbitrap 2V, positive-QTOF | splash10-03di-1900000000-56b961120be01ee88794 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 1-Methylxanthine Orbitrap 3V, positive-QTOF | splash10-03di-2900000000-a21b7d715ff67be12f30 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 1-Methylxanthine Orbitrap 4V, positive-QTOF | splash10-03di-5900000000-f069376ed73a3dc10f54 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 1-Methylxanthine Orbitrap 5V, positive-QTOF | splash10-0bt9-9500000000-627f0d2416135b20579b | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 1-Methylxanthine Orbitrap 6V, positive-QTOF | splash10-0a4i-9200000000-693e5185f1cfeede2a4f | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 1-Methylxanthine Orbitrap 7V, positive-QTOF | splash10-0a4i-9000000000-3a28092a50ffbb7dd36e | 2020-07-22 | HMDB team, MONA | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum |
IR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M-H]-) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+H]+) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+Na]+) | 2023-02-03 | FELIX lab | View Spectrum |
|
|---|
| Disease References | | Colorectal cancer |
|---|
- Brown DG, Rao S, Weir TL, O'Malia J, Bazan M, Brown RJ, Ryan EP: Metabolomics and metabolic pathway networks from human colorectal cancers, adjacent mucosa, and stool. Cancer Metab. 2016 Jun 6;4:11. doi: 10.1186/s40170-016-0151-y. eCollection 2016. [PubMed:27275383 ]
- Sinha R, Ahn J, Sampson JN, Shi J, Yu G, Xiong X, Hayes RB, Goedert JJ: Fecal Microbiota, Fecal Metabolome, and Colorectal Cancer Interrelations. PLoS One. 2016 Mar 25;11(3):e0152126. doi: 10.1371/journal.pone.0152126. eCollection 2016. [PubMed:27015276 ]
- Goedert JJ, Sampson JN, Moore SC, Xiao Q, Xiong X, Hayes RB, Ahn J, Shi J, Sinha R: Fecal metabolomics: assay performance and association with colorectal cancer. Carcinogenesis. 2014 Sep;35(9):2089-96. doi: 10.1093/carcin/bgu131. Epub 2014 Jul 18. [PubMed:25037050 ]
| | Asthma |
|---|
- Zydron M, Baranowski J, Baranowska I: Separation, pre-concentration, and HPLC analysis of methylxanthines in urine samples. J Sep Sci. 2004 Oct;27(14):1166-72. [PubMed:15537072 ]
|
|
|---|