| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected and Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2023-02-21 17:14:37 UTC |
|---|
| HMDB ID | HMDB0000223 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Oxalacetic acid |
|---|
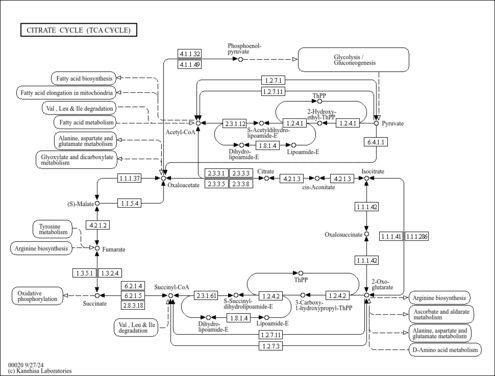
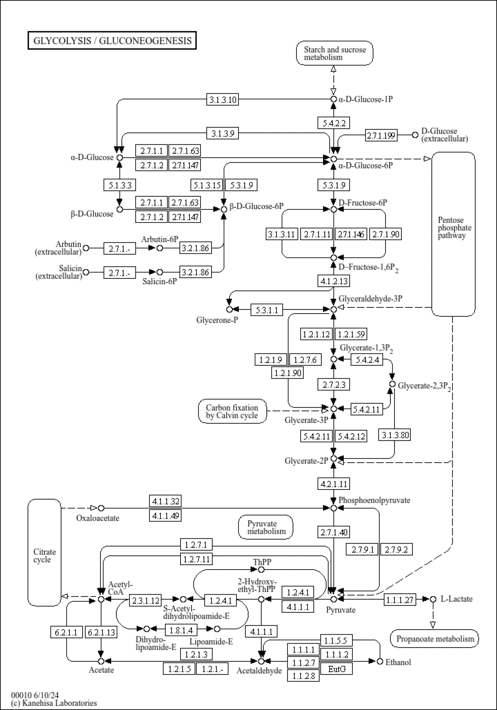
| Description | Oxalacetic acid, also known as oxaloacetic acid, keto-oxaloacetate or 2-oxobutanedioate, belongs to the class of organic compounds known as short-chain keto acids and derivatives. These are keto acids with an alkyl chain the contains less than 6 carbon atoms. Oxalacetic acid is a metabolic intermediate in many processes that occur in animals and plants. It takes part in gluconeogenesis, the urea cycle, the glyoxylate cycle, amino acid synthesis, fatty acid synthesis and the citric acid cycle. Oxalacetic acid exists in all living species, ranging from bacteria to plants to humans. Within humans, oxalacetic acid participates in a number of enzymatic reactions. In particular, oxalacetic acid is an intermediate of the citric acid cycle, where it reacts with acetyl-CoA to form citrate, catalyzed by citrate synthase. It is also involved in gluconeogenesis and the urea cycle. In gluconeogenesis oxaloacetate is decarboxylated and phosphorylated by phosphoenolpyruvate carboxykinase and becomes 2-phosphoenolpyruvate using guanosine triphosphate (GTP) as phosphate source. In the urea cycle, malate is acted on by malate dehydrogenase to become oxaloacetate, producing a molecule of NADH. After that, oxaloacetate can be recycled to aspartate, as this recycling maintains the flow of nitrogen into the cell. In mice, injections of oxalacetic acid have been shown to promote brain mitochondrial biogenesis, activate the insulin signaling pathway, reduce neuroinflammation and activate hippocampal neurogenesis (PMID: 25027327 ). Oxalacetic acid has also been reported to reduce hyperglycemia in type II diabetes and to extend longevity in C. elegans (PMID: 25027327 ). Outside of the human body, oxalacetic acid has been detected, but not quantified in, several different foods, such as Persian limes, lemon balms, wild rice, canola, and peanuts. This could make oxalacetic acid a potential biomarker for the consumption of these foods. |
|---|
| Structure | InChI=1S/C4H4O5/c5-2(4(8)9)1-3(6)7/h1H2,(H,6,7)(H,8,9) |
|---|
| Synonyms | | Value | Source |
|---|
| 2-Oxobutanedioic acid | ChEBI | | 2-Oxosuccinic acid | ChEBI | | 3-Carboxy-3-oxopropanoic acid | ChEBI | | Keto-succinic acid | ChEBI | | Ketosuccinic acid | ChEBI | | OAA | ChEBI | | Oxobutanedioic acid | ChEBI | | Oxosuccinic acid | ChEBI | | Oxaloacetic acid | Kegg | | Keto-oxaloacetate | Kegg | | 2-Oxobutanedioate | Generator | | 2-Oxosuccinate | Generator | | 3-Carboxy-3-oxopropanoate | Generator | | Keto-succinate | Generator | | Ketosuccinate | Generator | | Oxobutanedioate | Generator | | Oxosuccinate | Generator | | Oxaloacetate | Generator | | Keto-oxaloacetic acid | Generator | | Oxalacetate | Generator | | 2-Ketosuccinate | HMDB | | 2-Ketosuccinic acid | HMDB | | a-Ketosuccinate | HMDB | | a-Ketosuccinic acid | HMDB | | alpha-Ketosuccinate | HMDB | | alpha-Ketosuccinic acid | HMDB | | Oxaloethanoate | HMDB | | Oxaloethanoic acid | HMDB | | Acid, oxaloacetic | HMDB | | Acid, oxalacetic | HMDB | | 2 Ketosuccinic acid | HMDB | | 2 oxo Butanedioic acid | HMDB | | 2-oxo-Butanedioic acid | HMDB |
|
|---|
| Chemical Formula | C4H4O5 |
|---|
| Average Molecular Weight | 132.0716 |
|---|
| Monoisotopic Molecular Weight | 132.005873238 |
|---|
| IUPAC Name | 2-oxobutanedioic acid |
|---|
| Traditional Name | oxalacetate |
|---|
| CAS Registry Number | 328-42-7 |
|---|
| SMILES | OC(=O)CC(=O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C4H4O5/c5-2(4(8)9)1-3(6)7/h1H2,(H,6,7)(H,8,9) |
|---|
| InChI Key | KHPXUQMNIQBQEV-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as short-chain keto acids and derivatives. These are keto acids with an alkyl chain the contains less than 6 carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Keto acids and derivatives |
|---|
| Sub Class | Short-chain keto acids and derivatives |
|---|
| Direct Parent | Short-chain keto acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Beta-keto acid
- Short-chain keto acid
- Alpha-keto acid
- Beta-hydroxy ketone
- Dicarboxylic acid or derivatives
- 1,3-dicarbonyl compound
- Alpha-hydroxy ketone
- Ketone
- Carboxylic acid
- Carboxylic acid derivative
- Carbonyl group
- Organooxygen compound
- Organic oxide
- Organic oxygen compound
- Hydrocarbon derivative
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | 161 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 134 mg/mL | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Experimental Collision Cross Sections |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| Oxalacetic acid,1TMS,isomer #1 | C[Si](C)(C)OC(=O)CC(=O)C(=O)O | 1292.7 | Semi standard non polar | 33892256 | | Oxalacetic acid,1TMS,isomer #2 | C[Si](C)(C)OC(=O)C(=O)CC(=O)O | 1261.0 | Semi standard non polar | 33892256 | | Oxalacetic acid,1TMS,isomer #3 | C[Si](C)(C)OC(=CC(=O)O)C(=O)O | 1474.4 | Semi standard non polar | 33892256 | | Oxalacetic acid,2TMS,isomer #1 | C[Si](C)(C)OC(=O)CC(=O)C(=O)O[Si](C)(C)C | 1417.3 | Semi standard non polar | 33892256 | | Oxalacetic acid,2TMS,isomer #2 | C[Si](C)(C)OC(=O)C=C(O[Si](C)(C)C)C(=O)O | 1495.7 | Semi standard non polar | 33892256 | | Oxalacetic acid,2TMS,isomer #3 | C[Si](C)(C)OC(=O)C(=CC(=O)O)O[Si](C)(C)C | 1487.7 | Semi standard non polar | 33892256 | | Oxalacetic acid,3TMS,isomer #1 | C[Si](C)(C)OC(=O)C=C(O[Si](C)(C)C)C(=O)O[Si](C)(C)C | 1533.5 | Semi standard non polar | 33892256 | | Oxalacetic acid,3TMS,isomer #1 | C[Si](C)(C)OC(=O)C=C(O[Si](C)(C)C)C(=O)O[Si](C)(C)C | 1490.9 | Standard non polar | 33892256 | | Oxalacetic acid,3TMS,isomer #1 | C[Si](C)(C)OC(=O)C=C(O[Si](C)(C)C)C(=O)O[Si](C)(C)C | 1512.7 | Standard polar | 33892256 | | Oxalacetic acid,1TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)CC(=O)C(=O)O | 1557.1 | Semi standard non polar | 33892256 | | Oxalacetic acid,1TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OC(=O)C(=O)CC(=O)O | 1521.2 | Semi standard non polar | 33892256 | | Oxalacetic acid,1TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC(=CC(=O)O)C(=O)O | 1691.1 | Semi standard non polar | 33892256 | | Oxalacetic acid,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)CC(=O)C(=O)O[Si](C)(C)C(C)(C)C | 1852.2 | Semi standard non polar | 33892256 | | Oxalacetic acid,2TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OC(=O)C=C(O[Si](C)(C)C(C)(C)C)C(=O)O | 1940.0 | Semi standard non polar | 33892256 | | Oxalacetic acid,2TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC(=O)C(=CC(=O)O)O[Si](C)(C)C(C)(C)C | 1938.7 | Semi standard non polar | 33892256 | | Oxalacetic acid,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)C=C(O[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C | 2144.7 | Semi standard non polar | 33892256 | | Oxalacetic acid,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)C=C(O[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C | 2065.8 | Standard non polar | 33892256 | | Oxalacetic acid,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)C=C(O[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C | 1970.7 | Standard polar | 33892256 |
|
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental GC-MS | GC-MS Spectrum - Oxalacetic acid GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (Non-derivatized) | splash10-00dr-4900000000-9d943d40beaca3c602a1 | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Oxalacetic acid GC-MS (1 MEOX; 2 TMS) | splash10-007a-9210000000-020f60717e2ea79d1ccd | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Oxalacetic acid GC-MS (1 MEOX; 2 TMS) | splash10-000b-9540000000-a53f674cc98960834f88 | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Oxalacetic acid GC-MS (1 MEOX; 3 TMS) | splash10-001a-8940000000-40b790e06141d7180938 | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Oxalacetic acid GC-MS (Non-derivatized) | splash10-007a-9210000000-020f60717e2ea79d1ccd | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Oxalacetic acid GC-MS (Non-derivatized) | splash10-000b-9540000000-a53f674cc98960834f88 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Oxalacetic acid GC-MS (Non-derivatized) | splash10-001a-8940000000-40b790e06141d7180938 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Oxalacetic acid GC-EI-TOF (Non-derivatized) | splash10-0002-4930000000-7e995327b0b9c1af0914 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Oxalacetic acid GC-MS (Non-derivatized) - 70eV, Positive | splash10-000f-9100000000-6718417e7196063f9544 | 2016-09-22 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Oxalacetic acid GC-MS (2 TMS) - 70eV, Positive | splash10-00ds-9520000000-5a082ec8843f6fbc3b15 | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Oxalacetic acid GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Oxalacetic acid GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Oxalacetic acid GC-MS (TMS_1_1) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Oxalacetic acid GC-MS (TMS_1_2) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Oxalacetic acid GC-MS (TMS_1_3) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Oxalacetic acid GC-MS (TMS_2_2) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Oxalacetic acid GC-MS (TMS_2_3) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Oxalacetic acid GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Oxalacetic acid GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Oxalacetic acid GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Oxalacetic acid GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Oxalacetic acid GC-MS (TBDMS_2_2) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Oxalacetic acid GC-MS (TBDMS_2_3) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-0006-9000000000-f371299a07d43c23ff1d | 2015-03-01 | Not Available | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental LC-MS/MS | LC-MS/MS Spectrum - Oxalacetic acid Quattro_QQQ 10V, Negative-QTOF (Annotated) | splash10-000i-9000000000-0be675f3aa3e973393b7 | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Oxalacetic acid Quattro_QQQ 25V, Negative-QTOF (Annotated) | splash10-000f-9000000000-4fba97fcd7b0f2215f75 | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Oxalacetic acid Quattro_QQQ 40V, Negative-QTOF (Annotated) | splash10-0006-9000000000-9b1f4171aee6283a3cbd | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Oxalacetic acid , negative-QTOF | splash10-014i-0900000000-7c598fd9c78acb94dacd | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Oxalacetic acid QTOF 3V, positive-QTOF | splash10-014i-4900000000-e7803730ae7986a1b9fb | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Oxalacetic acid QTOF 4V, positive-QTOF | splash10-014r-5900000000-8e478338a7c0e65e62ba | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Oxalacetic acid QTOF 5V, positive-QTOF | splash10-014r-7900000000-f681763317e1ae86e691 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Oxalacetic acid QTOF 7V, positive-QTOF | splash10-00kr-9600000000-aa8adf9a96041dddbbdd | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Oxalacetic acid QTOF 10V, positive-QTOF | splash10-000i-9200000000-e62c47ca2bc492900178 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Oxalacetic acid QTOF 15V, positive-QTOF | splash10-000i-9000000000-5485ddd84a64ea0ca0b9 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Oxalacetic acid QTOF 17V, positive-QTOF | splash10-000i-9000000000-79521cb04a2b23e39c1e | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Oxalacetic acid QTOF 20V, positive-QTOF | splash10-000m-9000000000-601c8d2ec63f8b01a61e | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Oxalacetic acid QTOF 23V, positive-QTOF | splash10-000e-9000000000-13d369c58d8930958d92 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Oxalacetic acid QTOF 25V, positive-QTOF | splash10-0005-9000000000-df8387ef907fb71342eb | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Oxalacetic acid QTOF 27V, positive-QTOF | splash10-0005-9000000000-908644b5d7041346f582 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Oxalacetic acid QTOF 30V, positive-QTOF | splash10-0005-9000000000-ecca787a800164d0296f | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Oxalacetic acid QTOF 33V, positive-QTOF | splash10-0005-9000000000-180a7c51b1f93cb8fff2 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Oxalacetic acid QTOF 35V, positive-QTOF | splash10-0005-9000000000-709c49f609f5a711a288 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Oxalacetic acid QTOF 40V, positive-QTOF | splash10-0005-9000000000-aa103ab9beb03bd6eb22 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Oxalacetic acid 10V, Positive-QTOF | splash10-014i-3900000000-6f4e965a3f513bf8db13 | 2015-04-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Oxalacetic acid 20V, Positive-QTOF | splash10-014i-9600000000-8ae7abf60057088e14db | 2015-04-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Oxalacetic acid 40V, Positive-QTOF | splash10-01bc-9100000000-5724c215c46a4f568140 | 2015-04-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Oxalacetic acid 10V, Negative-QTOF | splash10-0019-8900000000-829898d0849b1fbf1c28 | 2015-04-25 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Oxalacetic acid 20V, Negative-QTOF | splash10-000i-9100000000-5a5ff8fb7cb52eebf0c8 | 2015-04-25 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Oxalacetic acid 40V, Negative-QTOF | splash10-0006-9000000000-657d1384d478ddc33b6d | 2015-04-25 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Experimental 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, experimental) | 2012-12-04 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Experimental 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | 2012-12-05 | Wishart Lab | View Spectrum | | Experimental 2D NMR | [1H, 13C] NMR Spectrum (2D, 800 MHz, experimental) | 2019-09-11 | Wishart Lab | View Spectrum |
IR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M-H]-) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+H]+) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+Na]+) | 2023-02-03 | FELIX lab | View Spectrum |
|
|---|
| General References | - Sweatman BC, Farrant RD, Holmes E, Ghauri FY, Nicholson JK, Lindon JC: 600 MHz 1H-NMR spectroscopy of human cerebrospinal fluid: effects of sample manipulation and assignment of resonances. J Pharm Biomed Anal. 1993 Aug;11(8):651-64. [PubMed:8257730 ]
- Zupke C, Sinskey AJ, Stephanopoulos G: Intracellular flux analysis applied to the effect of dissolved oxygen on hybridomas. Appl Microbiol Biotechnol. 1995 Dec;44(1-2):27-36. [PubMed:8579834 ]
- Efimov AS, Gulyi MF, Shcherbak AV, Dzvonkevich ND: [Levels of Krebs cycle metabolites in the blood and urine of patients with diabetes mellitus]. Probl Endokrinol (Mosk). 1983 Mar-Apr;29(2):10-4. [PubMed:6856592 ]
- el-Sharabasy MM: Observations on calcium oxalate stone formers. Br J Urol. 1992 Nov;70(5):474-7. [PubMed:1361403 ]
- Dworzak E, Grunicke H, Berger H, Jarosch E, Haas H, Hopfel I: [Pyruvate dehydrogenase deficiency in a child with persistent lactic acidosis]. J Clin Chem Clin Biochem. 1985 Jun;23(6):323-9. [PubMed:3926941 ]
- Koike K, Koike M: Fluorescent analysis of alpha-keto acids in serum and urine by high-performance liquid chromatography. Anal Biochem. 1984 Sep;141(2):481-7. [PubMed:6437276 ]
- Esenmo E, Chandramouli V, Schumann WC, Kumaran K, Wahren J, Landau BR: Use of 14CO2 in estimating rates of hepatic gluconeogenesis. Am J Physiol. 1992 Jul;263(1 Pt 1):E36-41. [PubMed:1322046 ]
- Petrarulo M, Facchini P, Cerelli E, Marangella M, Linari F: Citrate in urine determined with a new citrate lyase method. Clin Chem. 1995 Oct;41(10):1518-21. [PubMed:7586527 ]
- Sperl W, Maurer H, Dworschak E, Hopfel I, Hammerer I: [Lactic acid acidosis with mitochondrial myopathy due to a pyruvate dehydrogenase deficiency]. Padiatr Padol. 1985;20(1):55-67. [PubMed:3919358 ]
- Olubuyide IO, Festing MF, Chapman C, Higginson J, Whicher JT: Discriminant analysis of biochemical parameters in liver disease. Trop Gastroenterol. 1997 Jan-Mar;18(1):15-9. [PubMed:9197166 ]
- Rabinovich PD, Miliushkin PV: [Content of biological oxidation metabolites in the blood and urine of peptic ulcer patients]. Vopr Med Khim. 1979 Nov-Dec;25(6):755-8. [PubMed:516538 ]
- Schauenstein E, Kronberger L, Schaur RJ, Fink E, Georgiopulos E: [Malate and oxaloacetate levels in whole blood of patients with and without malignant tumor diseases]. Wien Klin Wochenschr. 1973 Jun 29;85(26):478-82. [PubMed:4717666 ]
- Allen RH, Stabler SP, Savage DG, Lindenbaum J: Elevation of 2-methylcitric acid I and II levels in serum, urine, and cerebrospinal fluid of patients with cobalamin deficiency. Metabolism. 1993 Aug;42(8):978-88. [PubMed:8345822 ]
- Wong LT, Davidson AG, Applegarth DA, Dimmick JE, Norman MG, Toone JR, Pirie G, Wong J: Biochemical and histologic pathology in an infant with cross-reacting material (negative) pyruvate carboxylase deficiency. Pediatr Res. 1986 Mar;20(3):274-9. [PubMed:3085060 ]
- Wilkins HM, Harris JL, Carl SM, E L, Lu J, Eva Selfridge J, Roy N, Hutfles L, Koppel S, Morris J, Burns JM, Michaelis ML, Michaelis EK, Brooks WM, Swerdlow RH: Oxaloacetate activates brain mitochondrial biogenesis, enhances the insulin pathway, reduces inflammation and stimulates neurogenesis. Hum Mol Genet. 2014 Dec 15;23(24):6528-41. doi: 10.1093/hmg/ddu371. Epub 2014 Jul 15. [PubMed:25027327 ]
|
|---|