| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Expected but not Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2021-09-14 15:45:40 UTC |
|---|
| HMDB ID | HMDB0000963 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 5-Methylthioribose 1-phosphate |
|---|
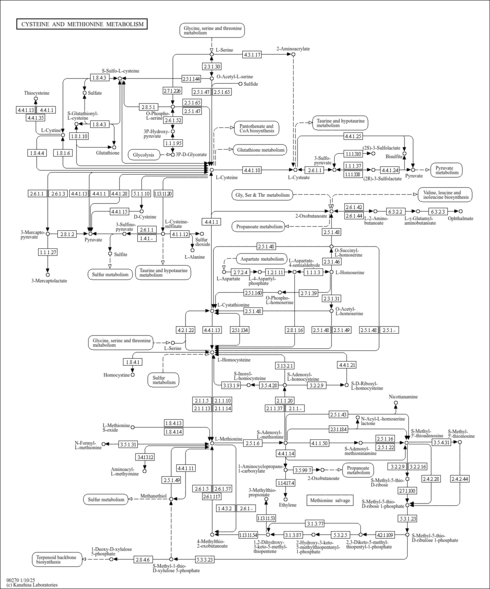
| Description | 5-Methylthioribose 1-phosphate belongs to the class of organic compounds known as pentoses. These are monosaccharides in which the carbohydrate moiety contains five carbon atoms. 5-Methylthioribose 1-phosphate is an intermediate in methionine biosynthesis. It is converted from 5'-deoxy-5'-methylthioadenosine by 5'-deoxy-5'-methylthioadenosine phosphorylase. Then it is converted to methionine (PMID: 2153115 ). In the methionine salvage pathway, 5-methylthioribose 1-phosphate isomerase (M1Pi) catalyzes the conversion of 5-methylthioribose 1-phosphate (MTR-1-P) into 5-methylthioribulose 1-phosphate (MTRu-1-P). |
|---|
| Structure | CSC[C@H]1O[C@H](OP(O)(O)=O)[C@H](O)[C@@H]1O InChI=1S/C6H13O7PS/c1-15-2-3-4(7)5(8)6(12-3)13-14(9,10)11/h3-8H,2H2,1H3,(H2,9,10,11)/t3-,4-,5-,6-/m1/s1 |
|---|
| Synonyms | | Value | Source |
|---|
| 5-S-Methyl-1-O-phosphono-5-thio-alpha-D-ribofuranose | ChEBI | | S-Methyl-5-thio-5-deoxy-D-ribose 1-phosphate | ChEBI | | S-Methyl-5-thio-D-ribose 1-phosphate | ChEBI | | 5-S-Methyl-1-O-phosphono-5-thio-a-D-ribofuranose | Generator | | 5-S-Methyl-1-O-phosphono-5-thio-α-D-ribofuranose | Generator | | S-Methyl-5-thio-5-deoxy-D-ribose 1-phosphoric acid | Generator | | S-Methyl-5-thio-D-ribose 1-phosphoric acid | Generator | | S-Methyl-5-thio-a-D-ribose 1-phosphate | Generator | | S-Methyl-5-thio-a-D-ribose 1-phosphoric acid | Generator | | S-Methyl-5-thio-alpha-D-ribose 1-phosphoric acid | Generator | | S-Methyl-5-thio-α-D-ribose 1-phosphate | Generator | | S-Methyl-5-thio-α-D-ribose 1-phosphoric acid | Generator | | S-Methyl-5-thio-alpha-D-ribose 1-phosphate | HMDB | | 1-PMTR | HMDB | | 1-Phospho-5-S-methylthio-alpha-D-ribofuranoside | HMDB | | 1-Phospho-5-S-methylthio-α-D-ribofuranoside | HMDB | | 1-Phospho-5-S-methylthioribose | HMDB | | 1-Phosphomethylthioribose | HMDB | | 5-Methylthio-D-ribose-1-phosphate | HMDB | | 5-Methylthioribose 1-phosphate | HMDB | | 5-Methylthioribose-1-phosphate | HMDB |
|
|---|
| Chemical Formula | C6H13O7PS |
|---|
| Average Molecular Weight | 260.202 |
|---|
| Monoisotopic Molecular Weight | 260.011959972 |
|---|
| IUPAC Name | {[(2R,3R,4S,5S)-3,4-dihydroxy-5-[(methylsulfanyl)methyl]oxolan-2-yl]oxy}phosphonic acid |
|---|
| Traditional Name | [(2R,3R,4S,5S)-3,4-dihydroxy-5-[(methylsulfanyl)methyl]oxolan-2-yl]oxyphosphonic acid |
|---|
| CAS Registry Number | 68134-74-7 |
|---|
| SMILES | CSC[C@H]1O[C@H](OP(O)(O)=O)[C@H](O)[C@@H]1O |
|---|
| InChI Identifier | InChI=1S/C6H13O7PS/c1-15-2-3-4(7)5(8)6(12-3)13-14(9,10)11/h3-8H,2H2,1H3,(H2,9,10,11)/t3-,4-,5-,6-/m1/s1 |
|---|
| InChI Key | JTFITTQBRJDSTL-KVTDHHQDSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as pentoses. These are monosaccharides in which the carbohydrate moiety contains five carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Pentoses |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pentose monosaccharide
- Monoalkyl phosphate
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Alkyl phosphate
- Tetrahydrofuran
- 1,2-diol
- Secondary alcohol
- Oxacycle
- Organoheterocyclic compound
- Dialkylthioether
- Sulfenyl compound
- Thioether
- Organic oxide
- Alcohol
- Hydrocarbon derivative
- Organosulfur compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Not Available |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Retention Times Underivatized| Chromatographic Method | Retention Time | Reference |
|---|
| Measured using a Waters Acquity ultraperformance liquid chromatography (UPLC) ethylene-bridged hybrid (BEH) C18 column (100 mm × 2.1 mm; 1.7 μmparticle diameter). Predicted by Afia on May 17, 2022. Predicted by Afia on May 17, 2022. | 2.48 minutes | 32390414 | | Predicted by Siyang on May 30, 2022 | 10.229 minutes | 33406817 | | Predicted by Siyang using ReTip algorithm on June 8, 2022 | 7.61 minutes | 32390414 | | AjsUoB = Accucore 150 Amide HILIC with 10mM Ammonium Formate, 0.1% Formic Acid | 450.4 seconds | 40023050 | | Fem_Long = Waters ACQUITY UPLC HSS T3 C18 with Water:MeOH and 0.1% Formic Acid | 567.8 seconds | 40023050 | | Fem_Lipids = Ascentis Express C18 with (60:40 water:ACN):(90:10 IPA:ACN) and 10mM NH4COOH + 0.1% Formic Acid | 311.2 seconds | 40023050 | | Life_Old = Waters ACQUITY UPLC BEH C18 with Water:(20:80 acetone:ACN) and 0.1% Formic Acid | 27.7 seconds | 40023050 | | Life_New = RP Waters ACQUITY UPLC HSS T3 C18 with Water:(30:70 MeOH:ACN) and 0.1% Formic Acid | 176.6 seconds | 40023050 | | RIKEN = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 63.2 seconds | 40023050 | | Eawag_XBridgeC18 = XBridge C18 3.5u 2.1x50 mm with Water:MeOH and 0.1% Formic Acid | 280.0 seconds | 40023050 | | BfG_NTS_RP1 =Agilent Zorbax Eclipse Plus C18 (2.1 mm x 150 mm, 3.5 um) with Water:ACN and 0.1% Formic Acid | 265.2 seconds | 40023050 | | HILIC_BDD_2 = Merck SeQuant ZIC-HILIC with ACN(0.1% formic acid):water(16 mM ammonium formate) | 828.1 seconds | 40023050 | | UniToyama_Atlantis = RP Waters Atlantis T3 (2.1 x 150 mm, 5 um) with ACN:Water and 0.1% Formic Acid | 659.8 seconds | 40023050 | | BDD_C18 = Hypersil Gold 1.9µm C18 with Water:ACN and 0.1% Formic Acid | 43.7 seconds | 40023050 | | UFZ_Phenomenex = Kinetex Core-Shell C18 2.6 um, 3.0 x 100 mm, Phenomenex with Water:MeOH and 0.1% Formic Acid | 625.6 seconds | 40023050 | | SNU_RIKEN_POS = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 182.7 seconds | 40023050 | | RPMMFDA = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 220.9 seconds | 40023050 | | MTBLS87 = Merck SeQuant ZIC-pHILIC column with ACN:Water and :ammonium carbonate | 811.1 seconds | 40023050 | | KI_GIAR_zic_HILIC_pH2_7 = Merck SeQuant ZIC-HILIC with ACN:Water and 0.1% FA | 510.0 seconds | 40023050 | | Meister zic-pHILIC pH9.3 = Merck SeQuant ZIC-pHILIC column with ACN:Water 5mM NH4Ac pH9.3 and 5mM ammonium acetate in water | 381.3 seconds | 40023050 |
Predicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| 5-Methylthioribose 1-phosphate,1TMS,isomer #1 | CSC[C@H]1O[C@H](OP(=O)(O)O)[C@H](O[Si](C)(C)C)[C@@H]1O | 2059.0 | Semi standard non polar | 33892256 | | 5-Methylthioribose 1-phosphate,1TMS,isomer #2 | CSC[C@H]1O[C@H](OP(=O)(O)O)[C@H](O)[C@@H]1O[Si](C)(C)C | 2054.6 | Semi standard non polar | 33892256 | | 5-Methylthioribose 1-phosphate,1TMS,isomer #3 | CSC[C@H]1O[C@H](OP(=O)(O)O[Si](C)(C)C)[C@H](O)[C@@H]1O | 2066.2 | Semi standard non polar | 33892256 | | 5-Methylthioribose 1-phosphate,2TMS,isomer #1 | CSC[C@H]1O[C@H](OP(=O)(O)O)[C@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C | 2052.1 | Semi standard non polar | 33892256 | | 5-Methylthioribose 1-phosphate,2TMS,isomer #2 | CSC[C@H]1O[C@H](OP(=O)(O)O[Si](C)(C)C)[C@H](O[Si](C)(C)C)[C@@H]1O | 2085.4 | Semi standard non polar | 33892256 | | 5-Methylthioribose 1-phosphate,2TMS,isomer #3 | CSC[C@H]1O[C@H](OP(=O)(O)O[Si](C)(C)C)[C@H](O)[C@@H]1O[Si](C)(C)C | 2073.5 | Semi standard non polar | 33892256 | | 5-Methylthioribose 1-phosphate,2TMS,isomer #4 | CSC[C@H]1O[C@H](OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)[C@H](O)[C@@H]1O | 2081.5 | Semi standard non polar | 33892256 | | 5-Methylthioribose 1-phosphate,3TMS,isomer #1 | CSC[C@H]1O[C@H](OP(=O)(O)O[Si](C)(C)C)[C@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C | 2078.9 | Semi standard non polar | 33892256 | | 5-Methylthioribose 1-phosphate,3TMS,isomer #1 | CSC[C@H]1O[C@H](OP(=O)(O)O[Si](C)(C)C)[C@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C | 2056.4 | Standard non polar | 33892256 | | 5-Methylthioribose 1-phosphate,3TMS,isomer #1 | CSC[C@H]1O[C@H](OP(=O)(O)O[Si](C)(C)C)[C@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C | 2574.4 | Standard polar | 33892256 | | 5-Methylthioribose 1-phosphate,3TMS,isomer #2 | CSC[C@H]1O[C@H](OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)[C@H](O[Si](C)(C)C)[C@@H]1O | 2092.3 | Semi standard non polar | 33892256 | | 5-Methylthioribose 1-phosphate,3TMS,isomer #2 | CSC[C@H]1O[C@H](OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)[C@H](O[Si](C)(C)C)[C@@H]1O | 2104.3 | Standard non polar | 33892256 | | 5-Methylthioribose 1-phosphate,3TMS,isomer #2 | CSC[C@H]1O[C@H](OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)[C@H](O[Si](C)(C)C)[C@@H]1O | 2459.7 | Standard polar | 33892256 | | 5-Methylthioribose 1-phosphate,3TMS,isomer #3 | CSC[C@H]1O[C@H](OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)[C@H](O)[C@@H]1O[Si](C)(C)C | 2098.1 | Semi standard non polar | 33892256 | | 5-Methylthioribose 1-phosphate,3TMS,isomer #3 | CSC[C@H]1O[C@H](OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)[C@H](O)[C@@H]1O[Si](C)(C)C | 2097.7 | Standard non polar | 33892256 | | 5-Methylthioribose 1-phosphate,3TMS,isomer #3 | CSC[C@H]1O[C@H](OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)[C@H](O)[C@@H]1O[Si](C)(C)C | 2426.3 | Standard polar | 33892256 | | 5-Methylthioribose 1-phosphate,4TMS,isomer #1 | CSC[C@H]1O[C@H](OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)[C@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C | 2126.4 | Semi standard non polar | 33892256 | | 5-Methylthioribose 1-phosphate,4TMS,isomer #1 | CSC[C@H]1O[C@H](OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)[C@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C | 2114.3 | Standard non polar | 33892256 | | 5-Methylthioribose 1-phosphate,4TMS,isomer #1 | CSC[C@H]1O[C@H](OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)[C@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C | 2329.2 | Standard polar | 33892256 | | 5-Methylthioribose 1-phosphate,1TBDMS,isomer #1 | CSC[C@H]1O[C@H](OP(=O)(O)O)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O | 2314.3 | Semi standard non polar | 33892256 | | 5-Methylthioribose 1-phosphate,1TBDMS,isomer #2 | CSC[C@H]1O[C@H](OP(=O)(O)O)[C@H](O)[C@@H]1O[Si](C)(C)C(C)(C)C | 2312.8 | Semi standard non polar | 33892256 | | 5-Methylthioribose 1-phosphate,1TBDMS,isomer #3 | CSC[C@H]1O[C@H](OP(=O)(O)O[Si](C)(C)C(C)(C)C)[C@H](O)[C@@H]1O | 2319.6 | Semi standard non polar | 33892256 | | 5-Methylthioribose 1-phosphate,2TBDMS,isomer #1 | CSC[C@H]1O[C@H](OP(=O)(O)O)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C | 2533.5 | Semi standard non polar | 33892256 | | 5-Methylthioribose 1-phosphate,2TBDMS,isomer #2 | CSC[C@H]1O[C@H](OP(=O)(O)O[Si](C)(C)C(C)(C)C)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O | 2543.4 | Semi standard non polar | 33892256 | | 5-Methylthioribose 1-phosphate,2TBDMS,isomer #3 | CSC[C@H]1O[C@H](OP(=O)(O)O[Si](C)(C)C(C)(C)C)[C@H](O)[C@@H]1O[Si](C)(C)C(C)(C)C | 2546.5 | Semi standard non polar | 33892256 | | 5-Methylthioribose 1-phosphate,2TBDMS,isomer #4 | CSC[C@H]1O[C@H](OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)[C@H](O)[C@@H]1O | 2540.2 | Semi standard non polar | 33892256 | | 5-Methylthioribose 1-phosphate,3TBDMS,isomer #1 | CSC[C@H]1O[C@H](OP(=O)(O)O[Si](C)(C)C(C)(C)C)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C | 2763.9 | Semi standard non polar | 33892256 | | 5-Methylthioribose 1-phosphate,3TBDMS,isomer #1 | CSC[C@H]1O[C@H](OP(=O)(O)O[Si](C)(C)C(C)(C)C)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C | 2639.3 | Standard non polar | 33892256 | | 5-Methylthioribose 1-phosphate,3TBDMS,isomer #1 | CSC[C@H]1O[C@H](OP(=O)(O)O[Si](C)(C)C(C)(C)C)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C | 2874.0 | Standard polar | 33892256 | | 5-Methylthioribose 1-phosphate,3TBDMS,isomer #2 | CSC[C@H]1O[C@H](OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O | 2754.2 | Semi standard non polar | 33892256 | | 5-Methylthioribose 1-phosphate,3TBDMS,isomer #2 | CSC[C@H]1O[C@H](OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O | 2645.5 | Standard non polar | 33892256 | | 5-Methylthioribose 1-phosphate,3TBDMS,isomer #2 | CSC[C@H]1O[C@H](OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O | 2789.7 | Standard polar | 33892256 | | 5-Methylthioribose 1-phosphate,3TBDMS,isomer #3 | CSC[C@H]1O[C@H](OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)[C@H](O)[C@@H]1O[Si](C)(C)C(C)(C)C | 2755.7 | Semi standard non polar | 33892256 | | 5-Methylthioribose 1-phosphate,3TBDMS,isomer #3 | CSC[C@H]1O[C@H](OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)[C@H](O)[C@@H]1O[Si](C)(C)C(C)(C)C | 2637.1 | Standard non polar | 33892256 | | 5-Methylthioribose 1-phosphate,3TBDMS,isomer #3 | CSC[C@H]1O[C@H](OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)[C@H](O)[C@@H]1O[Si](C)(C)C(C)(C)C | 2762.2 | Standard polar | 33892256 | | 5-Methylthioribose 1-phosphate,4TBDMS,isomer #1 | CSC[C@H]1O[C@H](OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C | 2980.2 | Semi standard non polar | 33892256 | | 5-Methylthioribose 1-phosphate,4TBDMS,isomer #1 | CSC[C@H]1O[C@H](OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C | 2796.8 | Standard non polar | 33892256 | | 5-Methylthioribose 1-phosphate,4TBDMS,isomer #1 | CSC[C@H]1O[C@H](OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C | 2753.3 | Standard polar | 33892256 |
|
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - 5-Methylthioribose 1-phosphate GC-MS (Non-derivatized) - 70eV, Positive | splash10-0002-9100000000-2488064947bfab511f5e | 2016-09-22 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 5-Methylthioribose 1-phosphate GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 5-Methylthioribose 1-phosphate GC-MS (TMS_1_1) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 5-Methylthioribose 1-phosphate GC-MS (TMS_1_2) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 5-Methylthioribose 1-phosphate GC-MS (TMS_1_3) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 5-Methylthioribose 1-phosphate GC-MS (TMS_2_1) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 5-Methylthioribose 1-phosphate GC-MS (TMS_2_2) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 5-Methylthioribose 1-phosphate GC-MS (TMS_2_3) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 5-Methylthioribose 1-phosphate GC-MS (TMS_2_4) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 5-Methylthioribose 1-phosphate GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 5-Methylthioribose 1-phosphate GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 5-Methylthioribose 1-phosphate GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 5-Methylthioribose 1-phosphate GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 5-Methylthioribose 1-phosphate GC-MS (TBDMS_2_2) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 5-Methylthioribose 1-phosphate GC-MS (TBDMS_2_3) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 5-Methylthioribose 1-phosphate GC-MS (TBDMS_2_4) - 70eV, Positive | Not Available | 2021-11-06 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 5-Methylthioribose 1-phosphate 10V, Positive-QTOF | splash10-0002-9340000000-81076180aee84a287f34 | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 5-Methylthioribose 1-phosphate 20V, Positive-QTOF | splash10-0002-9330000000-16cc1e6103ea1cca9414 | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 5-Methylthioribose 1-phosphate 40V, Positive-QTOF | splash10-001j-9200000000-e1e1c3a051f24524c0f3 | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 5-Methylthioribose 1-phosphate 10V, Negative-QTOF | splash10-0002-9010000000-6e1ba60f11f69a1cb95a | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 5-Methylthioribose 1-phosphate 20V, Negative-QTOF | splash10-004j-9000000000-138587ed5d90d657c9c1 | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 5-Methylthioribose 1-phosphate 40V, Negative-QTOF | splash10-004i-9000000000-41783131ed46fc1d13e5 | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 5-Methylthioribose 1-phosphate 10V, Positive-QTOF | splash10-03di-0590000000-7230baddac79c72027cc | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 5-Methylthioribose 1-phosphate 20V, Positive-QTOF | splash10-03di-5900000000-f9dacc6c82e4fa8c2fd2 | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 5-Methylthioribose 1-phosphate 40V, Positive-QTOF | splash10-01ot-9000000000-beff4b861a0056502418 | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 5-Methylthioribose 1-phosphate 10V, Negative-QTOF | splash10-0a4i-1090000000-ee3347d57dbc539ed78c | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 5-Methylthioribose 1-phosphate 20V, Negative-QTOF | splash10-004i-9000000000-0bc06e5e365bbcaeadc8 | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 5-Methylthioribose 1-phosphate 40V, Negative-QTOF | splash10-002b-9000000000-d539ffcaed3ac6a3dd0e | 2021-09-24 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum |
|
|---|
| General References | - Avila MA, Garcia-Trevijano ER, Lu SC, Corrales FJ, Mato JM: Methylthioadenosine. Int J Biochem Cell Biol. 2004 Nov;36(11):2125-30. [PubMed:15313459 ]
- Savarese TM, Ghoda LY, Dexter DL, Parks RE Jr: Conversion of 5'-deoxy-5'-methylthioadenosine and 5'-deoxy-5'-methylthioinosine to methionine in cultured human leukemic cells. Cancer Res. 1983 Oct;43(10):4699-702. [PubMed:6411330 ]
- Tisdale MJ: Methionine synthesis from 5'-methylthioadenosine by tumour cells. Biochem Pharmacol. 1983 Oct 1;32(19):2915-20. [PubMed:6626263 ]
- Savarese TM, Cannistra AJ, Parks RE Jr, Secrist JA 3rd, Shortnacy AT, Montgomery JA: 5'-deoxy-5'-methylthioadenosine phosphorylase--IV. Biological activity of 2-fluoroadenine-substituted 5'-deoxy-5'-methylthioadenosine analogs. Biochem Pharmacol. 1987 Jun 15;36(12):1881-93. [PubMed:3109431 ]
- Ghoda LY, Savarese TM, Dexter DL, Parks RE Jr, Trackman PC, Abeles RH: Characterization of a defect in the pathway for converting 5'-deoxy-5'-methylthioadenosine to methionine in a subline of a cultured heterogeneous human colon carcinoma. J Biol Chem. 1984 Jun 10;259(11):6715-9. [PubMed:6725268 ]
- Gianotti AJ, Tower PA, Sheley JH, Conte PA, Spiro C, Ferro AJ, Fitchen JH, Riscoe MK: Selective killing of Klebsiella pneumoniae by 5-trifluoromethylthioribose. Chemotherapeutic exploitation of the enzyme 5-methylthioribose kinase. J Biol Chem. 1990 Jan 15;265(2):831-7. [PubMed:2153115 ]
|
|---|