| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Expected but not Quantified |
|---|
| Creation Date | 2012-09-11 17:43:50 UTC |
|---|
| Update Date | 2022-03-07 02:53:01 UTC |
|---|
| HMDB ID | HMDB0031524 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Bromomethane |
|---|
| Description | Principally used as an insecticidal and nemacidal fumigant, especially for soil and agricultural produce. Also used as a solvent for extraction of plant oils The chemical compound bromomethane, commonly known as methyl bromide, is an organobromine compound with formula CH3Br. This colorless, odorless, nonflammable gas is produced both industrially and particularly biologically. It is a recognized ozone-depleting chemical. It was used extensively as a pesticide until being phased out by most countries in the early 2000s |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Brommethan | ChEBI | | CH3BR | ChEBI | | Embafume | ChEBI | | MeBr | ChEBI | | Methyl bromide | ChEBI | | Methylbromid | ChEBI | | Monobrommethan | ChEBI | | Monobromomethane | ChEBI | | Bercema | HMDB | | BMM | HMDB | | Brom-methan | HMDB | | Brom-O-gas | HMDB | | Brom-O-gas methyl bromide soil fumigant | HMDB | | Brom-O-gaz | HMDB | | Brom-O-sol | HMDB | | Bromo-methane | HMDB | | Bromometano | HMDB | | Bromur di metile | HMDB | | Bromure de methyle | HMDB | | Bromuro di metile | HMDB | | Broommethaan | HMDB | | Celfume | HMDB | | Chlorodibromomethane | HMDB | | Curafume | HMDB | | Dawson 100 | HMDB | | Detia gas ex-m | HMDB | | Dow fume MC2 | HMDB | | Dowfume | HMDB | | Dowfume MC-2 | HMDB | | Dowfume MC-2 fumigant | HMDB | | Dowfume MC-2 soil fumigant | HMDB | | Dowfume MC-2R | HMDB | | Dowfume MC-33 | HMDB | | Drexel plant bed gas | HMDB | | EDCO | HMDB | | Fumigant-1 | HMDB | | Fumigant-1 (obs.) | HMDB | | Halon 1001 | HMDB | | Haltox | HMDB | | Iscobrome | HMDB | | Kayafume | HMDB | | m-b-c Fumigant | HMDB | | MB | HMDB | | MBC Soil fumigant | HMDB | | MBC-33 Soil fumigant | HMDB | | MBX | HMDB | | Meb R | HMDB | | Merth-O-gas | HMDB | | Metafume | HMDB | | Meth-O-gas | HMDB | | Methogas | HMDB | | Methyl bromide as a structural fumigant | HMDB | | Methyl bromide rodent fumigant (with chloropicrin) | HMDB | | Methyl bromide, 14C-labeled | HMDB | | Methyl bromide, bsi, iso, jmaf | HMDB | | Methyl fume | HMDB | | Methylbromide | HMDB | | Metylu bromek | HMDB | | Pestmaster | HMDB | | Pestmaster (obs.) | HMDB | | Pestmaster soil fumigant-1 | HMDB | | Profume | HMDB | | Profume (obs.) | HMDB | | R 40b1 | HMDB | | Rfdfif@ | HMDB | | Rotox | HMDB | | Superior methyl bromide-2 | HMDB | | Terabol | HMDB | | Terr-O-cide II | HMDB | | Terr-O-gas | HMDB | | Terr-O-gas 100 | HMDB | | Terr-O-gas 67 | HMDB | | Tri-brom | HMDB | | Zytox | HMDB |
|
|---|
| Chemical Formula | CH3Br |
|---|
| Average Molecular Weight | 94.939 |
|---|
| Monoisotopic Molecular Weight | 93.941812743 |
|---|
| IUPAC Name | bromomethane |
|---|
| Traditional Name | methyl bromide |
|---|
| CAS Registry Number | 74-83-9 |
|---|
| SMILES | CBr |
|---|
| InChI Identifier | InChI=1S/CH3Br/c1-2/h1H3 |
|---|
| InChI Key | GZUXJHMPEANEGY-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as halomethanes. These are organic compounds in which at least one of the four hydrogen atoms of methane (CH4) are replaced by halogen atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organohalogen compounds |
|---|
| Class | Alkyl halides |
|---|
| Sub Class | Halomethanes |
|---|
| Direct Parent | Halomethanes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Halomethane
- Hydrocarbon derivative
- Organobromide
- Alkyl bromide
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Not Available | Not Available |
|---|
| Physical Properties |
|---|
| State | Liquid |
|---|
| Experimental Molecular Properties | |
|---|
| Experimental Chromatographic Properties | Not Available |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Kovats Retention IndicesUnderivatized| Metabolite | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| Bromomethane | CBr | 863.1 | Standard polar | 33892256 | | Bromomethane | CBr | 397.5 | Standard non polar | 33892256 | | Bromomethane | CBr | 406.8 | Semi standard non polar | 33892256 |
|
|---|
| Spectra |
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental GC-MS | GC-MS Spectrum - Bromomethane EI-B (Non-derivatized) | splash10-0007-9000000000-745f8b23626d21db04f3 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Bromomethane EI-B (Non-derivatized) | splash10-0007-9000000000-745f8b23626d21db04f3 | 2018-05-18 | HMDB team, MONA, MassBank | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Bromomethane GC-MS (Non-derivatized) - 70eV, Positive | splash10-0007-9000000000-7942af0aaf6970a66f15 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Bromomethane GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Bromomethane GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-0007-9000000000-169ac2e004c3655205b0 | 2014-09-20 | Not Available | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Bromomethane 10V, Positive-QTOF | splash10-0006-9000000000-489f85dffd44526b6faf | 2016-08-03 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Bromomethane 20V, Positive-QTOF | splash10-0006-9000000000-489f85dffd44526b6faf | 2016-08-03 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Bromomethane 40V, Positive-QTOF | splash10-0006-9000000000-489f85dffd44526b6faf | 2016-08-03 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Bromomethane 10V, Negative-QTOF | splash10-0006-9000000000-8cbdea03c774de7d15ba | 2016-08-03 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Bromomethane 20V, Negative-QTOF | splash10-0006-9000000000-8cbdea03c774de7d15ba | 2016-08-03 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Bromomethane 40V, Negative-QTOF | splash10-0006-9000000000-8cbdea03c774de7d15ba | 2016-08-03 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Bromomethane 10V, Positive-QTOF | splash10-0006-9000000000-7cb72c4b41f7b99ed261 | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Bromomethane 20V, Positive-QTOF | splash10-0006-9000000000-7cb72c4b41f7b99ed261 | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Bromomethane 40V, Positive-QTOF | splash10-0006-9000000000-7cb72c4b41f7b99ed261 | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Bromomethane 10V, Negative-QTOF | splash10-0006-9000000000-e800a553de932a2e14db | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Bromomethane 20V, Negative-QTOF | splash10-0006-9000000000-e800a553de932a2e14db | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Bromomethane 40V, Negative-QTOF | splash10-0006-9000000000-e800a553de932a2e14db | 2021-09-24 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-30 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-30 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-30 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-30 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-30 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-30 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-30 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-30 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-30 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-30 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-30 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-30 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-30 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-30 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-30 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-30 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-30 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-30 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-30 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-30 | Wishart Lab | View Spectrum |
|
|---|
| Biological Properties |
|---|
| Cellular Locations | |
|---|
| Biospecimen Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
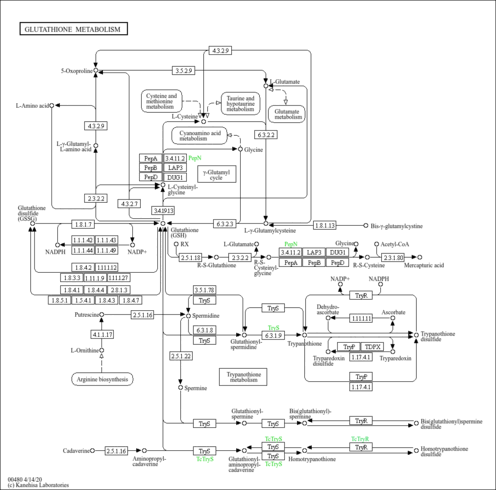
| Pathways | |
|---|
| Normal Concentrations |
|---|
| Not Available |
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| Associated Disorders and Diseases |
|---|
| Disease References | None |
|---|
| Associated OMIM IDs | None |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| Phenol Explorer Compound ID | Not Available |
|---|
| FooDB ID | FDB008126 |
|---|
| KNApSAcK ID | Not Available |
|---|
| Chemspider ID | 6083 |
|---|
| KEGG Compound ID | C18447 |
|---|
| BioCyc ID | CPD-12221 |
|---|
| BiGG ID | Not Available |
|---|
| Wikipedia Link | Bromomethane |
|---|
| METLIN ID | Not Available |
|---|
| PubChem Compound | 6323 |
|---|
| PDB ID | BMM |
|---|
| ChEBI ID | 39275 |
|---|
| Food Biomarker Ontology | Not Available |
|---|
| VMH ID | Not Available |
|---|
| MarkerDB ID | Not Available |
|---|
| Good Scents ID | rw1225721 |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| General References | - Zhao RS, Lao WJ, Xu XB: Headspace liquid-phase microextraction of trihalomethanes in drinking water and their gas chromatographic determination. Talanta. 2004 Mar 10;62(4):751-6. doi: 10.1016/j.talanta.2003.09.035. [PubMed:18969359 ]
- Mouly D, Joulin E, Rosin C, Beaudeau P, Zeghnoun A, Olszewski-Ortar A, Munoz JF, Welte B, Joyeux M, Seux R, Montiel A, Rodriguez MJ: Variations in trihalomethane levels in three French water distribution systems and the development of a predictive model. Water Res. 2010 Oct;44(18):5168-79. doi: 10.1016/j.watres.2010.06.028. Epub 2010 Jun 19. [PubMed:20663536 ]
- Graham EA: LATE POISONING WITH CHLOROFORM AND OTHER ALKYL HALIDES IN RELATIONSHIP TO THE HALOGEN ACIDS FORMED BY THEIR CHEMICAL DISSOCIATION. J Exp Med. 1915 Jul 1;22(1):48-75. [PubMed:19867902 ]
- Langfermann C, Klementz D, Sierts-Herrmann A, Poschadel B, Sagunski H, Hosch C, Horn K, Reichmuth C, Baur X: [Study on the potential impact of bromomethane on medicinal products following simulated container fumigation]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2007 Apr;50(4):492-9. [PubMed:17387439 ]
- FREW WD: Rectal tribrom-ethanol (avertin, bromethol) in eclamptic toxaemia. Can Med Assoc J. 1953 Sep;69(3):254-7. [PubMed:13082465 ]
- Catto C, Charest-Tardif G, Rodriguez M, Tardif R: Accounting for the impact of short-term variations in the levels of trihalomethane in drinking water on exposure assessment for epidemiological purposes. Part II: biological aspects. J Expo Sci Environ Epidemiol. 2013 Jan-Feb;23(1):60-6. doi: 10.1038/jes.2012.88. Epub 2012 Sep 12. [PubMed:22968351 ]
- Wang GS, Deng YC, Lin TF: Cancer risk assessment from trihalomethanes in drinking water. Sci Total Environ. 2007 Nov 15;387(1-3):86-95. Epub 2007 Aug 28. [PubMed:17727920 ]
- Narotsky MG, Best DS, McDonald A, Godin EA, Hunter ES 3rd, Simmons JE: Pregnancy loss and eye malformations in offspring of F344 rats following gestational exposure to mixtures of regulated trihalomethanes and haloacetic acids. Reprod Toxicol. 2011 Jan;31(1):59-65. doi: 10.1016/j.reprotox.2010.08.002. Epub 2010 Sep 17. [PubMed:20850520 ]
- Rodrigues PM, Esteves da Silva JC, Antunes MC: Factorial analysis of the trihalomethanes formation in water disinfection using chlorine. Anal Chim Acta. 2007 Jul 9;595(1-2):266-74. Epub 2006 Dec 23. [PubMed:17606009 ]
- Zsolnai T: [Tribrom-nitromethan--a new disinfectant of high efficiency (author's transl)]. Zentralbl Bakteriol Orig A. 1973 Oct;224(4):496-502. [PubMed:4150117 ]
- Saghir SA, Ghanayem BI, Schultz IR: Kinetics of trihalogenated acetic acid metabolism and isoform specificity in liver microsomes. Int J Toxicol. 2011 Oct;30(5):551-61. doi: 10.1177/1091581811414213. Epub 2011 Sep 20. [PubMed:21933969 ]
- Weaver WA, Li J, Wen Y, Johnston J, Blatchley MR, Blatchley ER 3rd: Volatile disinfection by-product analysis from chlorinated indoor swimming pools. Water Res. 2009 Jul;43(13):3308-18. doi: 10.1016/j.watres.2009.04.035. Epub 2009 May 3. [PubMed:19501873 ]
- (). Yannai, Shmuel. (2004) Dictionary of food compounds with CD-ROM: Additives, flavors, and ingredients. Boca Raton: Chapman & Hall/CRC.. .
|
|---|