| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected and Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2025-05-29 18:10:19 UTC |
|---|
| HMDB ID | HMDB0000479 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Methylhistidine |
|---|
| Description | 3-Methylhistidine, also known as 3-MHis, 3MH, pi-methylhistidine or pros-methylhistidine, belongs to the class of organic compounds known as histidine and derivatives. 3MH is also classified as a methylamino acid. Methylamino acids are primarily proteogenic amino acids (found in proteins) which have been methylated (in situ) on their side chains by various methyltransferase enzymes. Histidine can be methylated at either the N1 or N3 position of its imidazole ring, yielding the isomers 1-methylhistidine (1MH; also referred to as tau-methylhistidine, according to IUPAC) or 3-methylhistidine (3MH; pi-methylhistidine, according to IUPAC), respectively. There is considerable confusion with regard to the nomenclature of the methylated nitrogen atoms on the imidazole ring of histidine in histidine-containing proteins (such as actin and myosin) as well as histidine-containing peptides (such as anserine and ophidine/balenine). In particular, older literature (mostly prior to the year 2000) as well as most biochemists and nutrition scientists incorrectly number the imidazole nitrogen atom most proximal to the side chain beta-carbon as 1 or N1, while organic chemists correctly designate it as 3 or N3. As a result, biochemists and nutrition scientists historically designated anserine (Npi-methylated) as beta-alanyl-N1-methylhistidine (or beta-alanyl-1-methylhistidine), whereas according to standard IUPAC nomenclature, anserine is correctly named as beta-alanyl-N3-methylhistidine. As a result, for several decades, many papers incorrectly identified 1MH as a specific marker for dietary consumption or various pathophysiological effects when they really are referring to 3MH – and vice versa (PMID: 24137022 ). To help resolve this issue the IUPAC commission (PMID: 6743224 and IUPAC Compendium of Chemical Terminology, 2nd ed. (the 'Gold Book'). Compiled by A. D. McNaught and A. Wilkinson. Blackwell Scientific Publications, Oxford (1997)) revised the nomenclature for histidine and introduced the terms pi (for prox or pros – near) and tau (for tele – far) to label the imidazole nitrogens in histidine. Therefore, the pi nitrogen is the nitrogen closest to the side chain beta carbon (atom #3 or N3) while the tau nitrogen is most distant from the side chain beta carbon (atom #1 or N1). IUPAC’s goal is for the global community to refer to the molecule depicted here is as “pi-methylhistidine” with the hope that the archaic term, 3-methylhistidine will eventually disappear. Unfortunately, this has not happened and confusion still persists. Older versions of the HMDB (prior to 2022) as well as current versions of some databases, such as PubChem, KEGG, and UniProt, indicate that an acceptable synonym for 3MH is tau-methylhistidine or otherwise somehow equate 1MH and 3MH. This is incorrect and it continues to sow confusion. Indeed, a key paper that identified METTL9 as the enzyme responsible for pi-methylation of histidine in most vertebrates also incorrectly labeled the METTL9 product as 1MH (PMID: 33563959 ). Similarly, a key paper that identified METTL18 as the enzyme responsible for tau-methylation of histidine incorrectly labelled the METTL18 product as 3MH (PMID: 33693809 ). Likewise, many members of the biochemical/nutrition community still incorrectly refer to 1MH as pi-methlyhistidine and 3MH as tau-methylhistidine. This has led to even more confusion. To maintain consistency for this compound description, all papers cited herein that incorrectly refer to 3MH as 1MH and vice versa, will have their conclusions re-stated and the citation will be marked with the phrase “3MH/1MH switch”. 3MH is a free amino acid arising from the proteolysis of 3MH-containing proteins and peptides. It is not synthesized on its own, nor can it be incorporated into proteins as an amino acid. However, it can be incorporated into certain dipeptides through the action of the enzyme known as carnosine synthase I. 3MH can only be generated from histidine residues through the action of methyltransferases as a protein post-translational modification event. Histidine methylation on the 3- or pi site of histidine-containing proteins is mediated by only one known enzyme – METTL9. Recent discoveries have shown that 3MH is produced in essentially all vertebrates via the methyltransferase enzyme known as METTL9 (3MH/1MH switch - PMID: 33563959 ). METTL9 is a broad-specificity S-adenosylmethionine-mediated methyltransferase that mediates the formation of the majority of 3MH present in mammalian and other vertebrate proteomes. METTL9-catalyzed methylation requires a His-x-His (HxH) motif, where 'x' is a small amino acid consisting of A, N, G, S, or T. This H[ANGST]H or HxH motif is found in a number of abundant mammalian proteins such as ARMC6, S100A9, and NDUFB3 (3MH/1MH switch - PMID: 33563959 ). In addition to these pi-His-methylated proteins, a specialized dipeptide called anserine (called beta-alanyl-3-methyl-L-histidine) that consists of beta-alanine and 3MH is also known and particularly well studied (PMID: 24137022 ). This methylated analog of carnosine, which is naturally produced in the liver via the enzyme carnosine synthase I (PMID: 20097752 ), is especially abundant in the skeletal muscles and brains of mammals, birds, and fish (PMID: 24137022 ). Anserine, like its homologs ophidine and carnosine, is believed to act as a pH buffer (for lactic acid generated by muscles), an antiglycating agent, and an antioxidant. Neither ophidine nor anserine are produced in humans, with humans being the only vertebrate not producing (or producing very little) methylated histidine versions of carnosine (PMID: 24137022 ). Because of its abundance in some muscle-related proteins but especially because of the high abundance of anserine found in poultry and fish, 3MH has been found to be a good biomarker for the consumption of meat (PMID: 21527577 ). Dietary studies have shown that general poultry consumption (p-trend = 0.0006) and especially chicken consumption (p-trend = 0.0003) are associated with increased levels of 3MH in human plasma (PMID: 30018457 ). The consumption of fish, especially salmon and cod, has also been shown to increase the levels of 3MH in serum and urine (3MH/1MH switch PMID: 31401679 ). As a general rule, urinary 3MH is associated with white meat intake (p< 0.001), whereas urinary 1MH is associated with red meat intake (p< 0.001) (3MH/1MH switch - PMID: 34091671 ). |
|---|
| Structure | [H][C@](N)(CC1=CN=CN1C)C(O)=O InChI=1S/C7H11N3O2/c1-10-4-9-3-5(10)2-6(8)7(11)12/h3-4,6H,2,8H2,1H3,(H,11,12)/t6-/m0/s1 |
|---|
| Synonyms | | Value | Source |
|---|
| (2S)-2-Amino-3-(1-methyl-1H-imidazol-5-yl)propanoic acid | ChEBI | | 3-Methyl-L-histidine | ChEBI | | N-pros-Methyl-L-histidine | ChEBI | | (2S)-2-Amino-3-(1-methyl-1H-imidazol-5-yl)propanoate | Generator | | 3-N-Methyl-L-histidine | HMDB | | L-3-Methylhistidine | HMDB | | N(pros)-Methyl-L-histidine | HMDB | | N3-Methyl-L-histidine | HMDB | | 3-Methylhistidine hydride | HMDB | | 3-Methylhistidine dihydrochloride | HMDB | | 3-Methylhistidine | ChEBI | | Pi methylhistidine | HMDB | | N(Pi)-methylhistidine | HMDB | | N Pi-methylhistidine | HMDB |
|
|---|
| Chemical Formula | C7H11N3O2 |
|---|
| Average Molecular Weight | 169.1811 |
|---|
| Monoisotopic Molecular Weight | 169.085126611 |
|---|
| IUPAC Name | (2S)-2-amino-3-(1-methyl-1H-imidazol-5-yl)propanoic acid |
|---|
| Traditional Name | 3,methylhistidine |
|---|
| CAS Registry Number | 368-16-1 |
|---|
| SMILES | [H][C@](N)(CC1=CN=CN1C)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C7H11N3O2/c1-10-4-9-3-5(10)2-6(8)7(11)12/h3-4,6H,2,8H2,1H3,(H,11,12)/t6-/m0/s1 |
|---|
| InChI Key | JDHILDINMRGULE-LURJTMIESA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as histidine and derivatives. Histidine and derivatives are compounds containing cysteine or a derivative thereof resulting from reaction of cysteine at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Histidine and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Histidine or derivatives
- Alpha-amino acid
- L-alpha-amino acid
- Imidazolyl carboxylic acid derivative
- Aralkylamine
- N-substituted imidazole
- Azole
- Imidazole
- Heteroaromatic compound
- Amino acid
- Carboxylic acid
- Azacycle
- Organoheterocyclic compound
- Monocarboxylic acid or derivatives
- Organic nitrogen compound
- Organonitrogen compound
- Organooxygen compound
- Primary amine
- Primary aliphatic amine
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Carbonyl group
- Organic oxygen compound
- Amine
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 200 mg/mL at 25 °C | BEILSTEIN | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Experimental Collision Cross Sections |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Retention Times Underivatized| Chromatographic Method | Retention Time | Reference |
|---|
| Measured using a Waters Acquity ultraperformance liquid chromatography (UPLC) ethylene-bridged hybrid (BEH) C18 column (100 mm × 2.1 mm; 1.7 μmparticle diameter). Predicted by Afia on May 17, 2022. Predicted by Afia on May 17, 2022. | 1.57 minutes | 32390414 | | Predicted by Siyang on May 30, 2022 | 9.0663 minutes | 33406817 | | Predicted by Siyang using ReTip algorithm on June 8, 2022 | 8.66 minutes | 32390414 | | AjsUoB = Accucore 150 Amide HILIC with 10mM Ammonium Formate, 0.1% Formic Acid | 441.4 seconds | 40023050 | | Fem_Long = Waters ACQUITY UPLC HSS T3 C18 with Water:MeOH and 0.1% Formic Acid | 367.2 seconds | 40023050 | | Fem_Lipids = Ascentis Express C18 with (60:40 water:ACN):(90:10 IPA:ACN) and 10mM NH4COOH + 0.1% Formic Acid | 297.3 seconds | 40023050 | | Life_Old = Waters ACQUITY UPLC BEH C18 with Water:(20:80 acetone:ACN) and 0.1% Formic Acid | 39.5 seconds | 40023050 | | Life_New = RP Waters ACQUITY UPLC HSS T3 C18 with Water:(30:70 MeOH:ACN) and 0.1% Formic Acid | 183.5 seconds | 40023050 | | RIKEN = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 84.0 seconds | 40023050 | | Eawag_XBridgeC18 = XBridge C18 3.5u 2.1x50 mm with Water:MeOH and 0.1% Formic Acid | 301.4 seconds | 40023050 | | BfG_NTS_RP1 =Agilent Zorbax Eclipse Plus C18 (2.1 mm x 150 mm, 3.5 um) with Water:ACN and 0.1% Formic Acid | 223.0 seconds | 40023050 | | HILIC_BDD_2 = Merck SeQuant ZIC-HILIC with ACN(0.1% formic acid):water(16 mM ammonium formate) | 989.7 seconds | 40023050 | | UniToyama_Atlantis = RP Waters Atlantis T3 (2.1 x 150 mm, 5 um) with ACN:Water and 0.1% Formic Acid | 563.0 seconds | 40023050 | | BDD_C18 = Hypersil Gold 1.9µm C18 with Water:ACN and 0.1% Formic Acid | 40.8 seconds | 40023050 | | UFZ_Phenomenex = Kinetex Core-Shell C18 2.6 um, 3.0 x 100 mm, Phenomenex with Water:MeOH and 0.1% Formic Acid | 610.5 seconds | 40023050 | | SNU_RIKEN_POS = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 199.9 seconds | 40023050 | | RPMMFDA = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 322.0 seconds | 40023050 | | MTBLS87 = Merck SeQuant ZIC-pHILIC column with ACN:Water and :ammonium carbonate | 764.5 seconds | 40023050 | | KI_GIAR_zic_HILIC_pH2_7 = Merck SeQuant ZIC-HILIC with ACN:Water and 0.1% FA | 712.6 seconds | 40023050 | | Meister zic-pHILIC pH9.3 = Merck SeQuant ZIC-pHILIC column with ACN:Water 5mM NH4Ac pH9.3 and 5mM ammonium acetate in water | 412.3 seconds | 40023050 |
Predicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| 3-Methylhistidine,1TMS,isomer #1 | CN1C=NC=C1C[C@H](N)C(=O)O[Si](C)(C)C | 1761.5 | Semi standard non polar | 33892256 | | 3-Methylhistidine,1TMS,isomer #2 | CN1C=NC=C1C[C@H](N[Si](C)(C)C)C(=O)O | 1862.9 | Semi standard non polar | 33892256 | | 3-Methylhistidine,2TMS,isomer #1 | CN1C=NC=C1C[C@H](N[Si](C)(C)C)C(=O)O[Si](C)(C)C | 1861.2 | Semi standard non polar | 33892256 | | 3-Methylhistidine,2TMS,isomer #1 | CN1C=NC=C1C[C@H](N[Si](C)(C)C)C(=O)O[Si](C)(C)C | 1797.2 | Standard non polar | 33892256 | | 3-Methylhistidine,2TMS,isomer #1 | CN1C=NC=C1C[C@H](N[Si](C)(C)C)C(=O)O[Si](C)(C)C | 2267.1 | Standard polar | 33892256 | | 3-Methylhistidine,2TMS,isomer #2 | CN1C=NC=C1C[C@@H](C(=O)O)N([Si](C)(C)C)[Si](C)(C)C | 1995.6 | Semi standard non polar | 33892256 | | 3-Methylhistidine,2TMS,isomer #2 | CN1C=NC=C1C[C@@H](C(=O)O)N([Si](C)(C)C)[Si](C)(C)C | 1919.9 | Standard non polar | 33892256 | | 3-Methylhistidine,2TMS,isomer #2 | CN1C=NC=C1C[C@@H](C(=O)O)N([Si](C)(C)C)[Si](C)(C)C | 2428.2 | Standard polar | 33892256 | | 3-Methylhistidine,3TMS,isomer #1 | CN1C=NC=C1C[C@@H](C(=O)O[Si](C)(C)C)N([Si](C)(C)C)[Si](C)(C)C | 2044.1 | Semi standard non polar | 33892256 | | 3-Methylhistidine,3TMS,isomer #1 | CN1C=NC=C1C[C@@H](C(=O)O[Si](C)(C)C)N([Si](C)(C)C)[Si](C)(C)C | 1954.1 | Standard non polar | 33892256 | | 3-Methylhistidine,3TMS,isomer #1 | CN1C=NC=C1C[C@@H](C(=O)O[Si](C)(C)C)N([Si](C)(C)C)[Si](C)(C)C | 2157.3 | Standard polar | 33892256 | | 3-Methylhistidine,1TBDMS,isomer #1 | CN1C=NC=C1C[C@H](N)C(=O)O[Si](C)(C)C(C)(C)C | 2018.0 | Semi standard non polar | 33892256 | | 3-Methylhistidine,1TBDMS,isomer #2 | CN1C=NC=C1C[C@H](N[Si](C)(C)C(C)(C)C)C(=O)O | 2110.9 | Semi standard non polar | 33892256 | | 3-Methylhistidine,2TBDMS,isomer #1 | CN1C=NC=C1C[C@H](N[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C | 2304.8 | Semi standard non polar | 33892256 | | 3-Methylhistidine,2TBDMS,isomer #1 | CN1C=NC=C1C[C@H](N[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C | 2268.3 | Standard non polar | 33892256 | | 3-Methylhistidine,2TBDMS,isomer #1 | CN1C=NC=C1C[C@H](N[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C | 2437.1 | Standard polar | 33892256 | | 3-Methylhistidine,2TBDMS,isomer #2 | CN1C=NC=C1C[C@@H](C(=O)O)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2437.4 | Semi standard non polar | 33892256 | | 3-Methylhistidine,2TBDMS,isomer #2 | CN1C=NC=C1C[C@@H](C(=O)O)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2357.5 | Standard non polar | 33892256 | | 3-Methylhistidine,2TBDMS,isomer #2 | CN1C=NC=C1C[C@@H](C(=O)O)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2521.6 | Standard polar | 33892256 | | 3-Methylhistidine,3TBDMS,isomer #1 | CN1C=NC=C1C[C@@H](C(=O)O[Si](C)(C)C(C)(C)C)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2667.8 | Semi standard non polar | 33892256 | | 3-Methylhistidine,3TBDMS,isomer #1 | CN1C=NC=C1C[C@@H](C(=O)O[Si](C)(C)C(C)(C)C)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2596.9 | Standard non polar | 33892256 | | 3-Methylhistidine,3TBDMS,isomer #1 | CN1C=NC=C1C[C@@H](C(=O)O[Si](C)(C)C(C)(C)C)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2457.8 | Standard polar | 33892256 |
|
|---|
| Spectra |
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - Methylhistidine GC-MS (Non-derivatized) - 70eV, Positive | splash10-00dm-9500000000-ab7d6324146b8775fe50 | 2016-09-22 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Methylhistidine GC-MS (1 TMS) - 70eV, Positive | splash10-00fr-9400000000-42443c78fdfca9db1926 | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Methylhistidine GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Methylhistidine GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Methylhistidine GC-MS (TMS_1_2) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Methylhistidine GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Methylhistidine GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental LC-MS/MS | LC-MS/MS Spectrum - Methylhistidine Quattro_QQQ 10V, Positive-QTOF (Annotated) | splash10-014i-2900000000-15f0e43eb0d06e7f3ef3 | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Methylhistidine Quattro_QQQ 25V, Positive-QTOF (Annotated) | splash10-0002-9200000000-5a1ab4d052e6ba4a76e4 | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Methylhistidine Quattro_QQQ 40V, Positive-QTOF (Annotated) | splash10-0002-9000000000-c1951c88c84885804fb6 | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Methylhistidine LC-ESI-QQ (API3000, Applied Biosystems) 10V, Positive-QTOF | splash10-00di-0900000000-4e0fea0fe9b1142db07b | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Methylhistidine LC-ESI-QQ (API3000, Applied Biosystems) 20V, Positive-QTOF | splash10-0592-4900000000-ee1bce969d650e9fcfbe | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Methylhistidine LC-ESI-QQ (API3000, Applied Biosystems) 30V, Positive-QTOF | splash10-0002-9200000000-1ad733cdcfa4db4fe3b9 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Methylhistidine LC-ESI-QQ (API3000, Applied Biosystems) 40V, Positive-QTOF | splash10-0002-9000000000-a87ff5cc0642ea3af69c | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Methylhistidine LC-ESI-QQ (API3000, Applied Biosystems) 50V, Positive-QTOF | splash10-0002-9000000000-28a73708493f968c2ac6 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Methylhistidine LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Positive-QTOF | splash10-0002-9800000000-6565ccf96757ef3bc08f | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Methylhistidine LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Negative-QTOF | splash10-014i-0900000000-3cbd02f5b6c4e6e64eb9 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Methylhistidine LC-ESI-IT , negative-QTOF | splash10-0udi-0900000000-e6ce7548fd6f09e2098f | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Methylhistidine LC-ESI-QTOF , negative-QTOF | splash10-014i-0900000000-3cbd02f5b6c4e6e64eb9 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Methylhistidine LC-ESI-QQ , positive-QTOF | splash10-00di-0900000000-2d351351f285b2188710 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Methylhistidine LC-ESI-QQ , positive-QTOF | splash10-0592-4900000000-ee1bce969d650e9fcfbe | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Methylhistidine LC-ESI-QQ , positive-QTOF | splash10-0002-9200000000-1ad733cdcfa4db4fe3b9 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Methylhistidine LC-ESI-QQ , positive-QTOF | splash10-0002-9000000000-2ed34e7b458d9f8c092f | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Methylhistidine LC-ESI-QQ , positive-QTOF | splash10-0002-9000000000-c1b8a62adc15b80955c3 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Methylhistidine LC-ESI-QTOF , positive-QTOF | splash10-0002-9800000000-6565ccf96757ef3bc08f | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Methylhistidine 20V, Positive-QTOF | splash10-00di-6900000000-acc4a08855924a8bf5a1 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Methylhistidine 10V, Positive-QTOF | splash10-00di-0900000000-1ca24429b22e547d10d9 | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Methylhistidine 20V, Positive-QTOF | splash10-00di-1900000000-4cafc4698b6e3649ce7a | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Methylhistidine 40V, Positive-QTOF | splash10-0002-9300000000-ef0c8b716935df30c508 | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Methylhistidine 10V, Negative-QTOF | splash10-014i-1900000000-8a91a6a64bb07285c36d | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Methylhistidine 20V, Negative-QTOF | splash10-01b9-4900000000-4ad91c6f55caf69120e9 | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Methylhistidine 40V, Negative-QTOF | splash10-05di-9300000000-ea67f38340a4b7ef8e11 | 2016-09-12 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Experimental 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, experimental) | 2012-12-04 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Experimental 1D NMR | 13C NMR Spectrum (1D, 400 MHz, H2O, experimental) | 2021-10-10 | Wishart Lab | View Spectrum | | Experimental 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | 2012-12-05 | Wishart Lab | View Spectrum |
IR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M-H]-) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+H]+) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+Na]+) | 2023-02-03 | FELIX lab | View Spectrum |
|
|---|
| Biological Properties |
|---|
| Cellular Locations | |
|---|
| Biospecimen Locations | - Blood
- Cerebrospinal Fluid (CSF)
- Feces
- Saliva
- Urine
|
|---|
| Tissue Locations | |
|---|
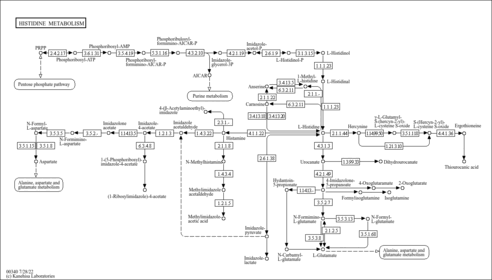
| Pathways | |
|---|
| Normal Concentrations |
|---|
| |
| Blood | Detected and Quantified | 3.0 (2.7 - 5.9) uM | Adult (>18 years old) | Not Specified | Normal | | details | | Blood | Detected and Quantified | 22.09 ± 14.41 uM | Adult (>18 years old) | Both | Normal | | details | | Blood | Detected and Quantified | 20.08 ± 11.86 uM | Adult (>18 years old) | Both | Normal | | details | | Blood | Detected and Quantified | 21.32 ± 12.09 uM | Adult (>18 years old) | Both | Normal | | details | | Blood | Detected and Quantified | 20.74 ± 13.18 uM | Adult (>18 years old) | Both | Normal | | details | | Blood | Detected and Quantified | 19.54 ± 11.89 uM | Adult (>18 years old) | Both | Normal | | details | | Blood | Detected and Quantified | 17.98 ± 9.51 uM | Adult (>18 years old) | Both | Normal | | details | | Blood | Detected and Quantified | 20.39 ± 13.62 uM | Adult (>18 years old) | Both | Normal | | details | | Blood | Detected and Quantified | 19.9 ± 12.64 uM | Adult (>18 years old) | Both | Normal | | details | | Blood | Detected and Quantified | 22.27 ± 10.29 uM | Adult (>18 years old) | Both | Normal | | details | | Blood | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Blood | Detected and Quantified | 2.7 (0.0-5.9) uM | Adult (>18 years old) | Both | Normal | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 3.82 +/- 2.43 uM | Adult (>18 years old) | Not Specified | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Not Specified | Both | Normal | | details | | Saliva | Detected and Quantified | 0.126 +/- 0.139 uM | Adult (>18 years old) | Female | Normal | | details | | Saliva | Detected and Quantified | 0.00950 +/- 0.0494 uM | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected and Quantified | 27.68 +/- 28.12 uM | Adult (>18 years old) | Both | Normal | | details | | Saliva | Detected and Quantified | 0.0982 +/- 0.128 uM | Adult (>18 years old) | Female | Normal | | details | | Saliva | Detected and Quantified | 0.134 +/- 0.110 uM | Adult (>18 years old) | Female | Normal | | details | | Urine | Detected and Quantified | 69.27 umol/mmol creatinine | Adult (>18 years old) | Male | Normal | | details | | Urine | Detected and Quantified | 16.5 (2.8-59.8) umol/mmol creatinine | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected and Quantified | 5.26 umol/mmol creatinine | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected and Quantified | 42.71 (31.68-56.08) umol/mmol creatinine | Newborn (0-30 days old) | Both | Normal | | details | | Urine | Detected and Quantified | 42.76 +/- 22.84 umol/mmol creatinine | Infant (0-1 year old) | Both | Normal | | details | | Urine | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected and Quantified | 7.92-27.83 umol/mmol creatinine | Adult (>18 years old) | Not Specified | Normal | | details | | Urine | Detected and Quantified | 5.32-29.64 umol/mmol creatinine | Adolescent (13-18 years old) | Not Specified | Normal | | details | | Urine | Detected and Quantified | 12.5 +/- 4.2 umol/mmol creatinine | Adult (>18 years old) | Not Specified | Normal | | details | | Urine | Detected and Quantified | 8.71-29.41 umol/mmol creatinine | Children (1-13 years old) | Not Specified | Normal | | details | | Urine | Detected and Quantified | 6.33-35.75 umol/mmol creatinine | Children (1-13 years old) | Not Specified | Normal | | details | | Urine | Detected and Quantified | 9.73-37.33 umol/mmol creatinine | Children (1-13 years old) | Not Specified | Normal | | details | | Urine | Detected and Quantified | 15.806 +/- 5.404 umol/mmol creatinine | Adult (>18 years old) | Female | Normal | | details | | Urine | Detected and Quantified | 44.944 +/- 41.318 umol/mmol creatinine | Children (1 - 13 years old) | Not Specified | Normal | | details | | Urine | Detected and Quantified | 5.8-50 umol/mmol creatinine | Adult (>18 years old) | Female | Normal | | details | | Urine | Detected and Quantified | 5.4-35 umol/mmol creatinine | Adult (>18 years old) | Male | Normal | | details | | Urine | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Urine | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected and Quantified | 17.977 +/- 5.902 umol/mmol creatinine | Adult (>18 years old) | Female | Normal | | details | | Urine | Detected and Quantified | 18.191 +/- 7.0323 umol/mmol creatinine | Adult (>18 years old) | Male | Normal | | details | | Urine | Detected and Quantified | 9.95-39.59 umol/mmol creatinine | Infant (0-1 year old) | Not Specified | Normal | | details | | Urine | Detected and Quantified | 13.556 +/- 2.487 umol/mmol creatinine | Adult (>18 years old) | Male | Normal | | details | | Urine | Detected and Quantified | 18.203 +/- 6.512 umol/mmol creatinine | Adult (>18 years old) | Female | Normal | | details | | Urine | Detected and Quantified | 17.705 +/- 6.00349 umol/mmol creatinine | Adult (>18 years old) | Male | Normal | | details | | Urine | Detected and Quantified | 16.0206 +/- 4.895 umol/mmol creatinine | Adult (>18 years old) | Female | Normal | | details | | Urine | Detected and Quantified | 17.0268 +/- 1.572 umol/mmol creatinine | Adult (>18 years old) | Male | Normal | | details | | Urine | Detected and Quantified | 32.79 +/- 20.35 umol/mmol creatinine | Newborn (0-30 days old) | Both | Normal | | details | | Urine | Detected and Quantified | 39.57 +/- 15.83 umol/mmol creatinine | Newborn (0-30 days old) | Both | Normal | | details | | Urine | Detected and Quantified | 39.57 +/- 23.74 umol/mmol creatinine | Newborn (0-30 days old) | Both | Normal | | details |
|
|---|
| Abnormal Concentrations |
|---|
| |
| Blood | Detected and Quantified | 5.16 +/- 1.27 uM | Elderly (>65 years old) | Both | Alzheimer's disease | | details | | Blood | Detected and Quantified | 13.0 +/- 10.4 uM | Adult (>18 years old) | Both | Kidney disease | | details | | Blood | Detected and Quantified | 23.7 +/- 18.6 uM | Adult (>18 years old) | Both | Kidney disease | | details | | Blood | Detected and Quantified | 50.7 (12.9) uM | Adult (>18 years old) | Female | Early preeclampsia | | details | | Blood | Detected and Quantified | 50.0 (14.6) uM | Adult (>18 years old) | Female | Pregnancy | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 2.48 +/- 1.58 uM | Adult (>18 years old) | Not Specified | Alzheimer's disease | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Colorectal cancer | | details | | Urine | Detected and Quantified | 11.6 +/- 6.6 umol/mmol creatinine | Adult (>18 years old) | Not Specified | Diabetes mellitus | | details | | Urine | Detected and Quantified | 16.0 +/- 9.0 umol/mmol creatinine | Adult (>18 years old) | Not Specified | Obesity | | details | | Urine | Detected and Quantified | 24.0 +/- 1.7 umol/mmol creatinine | Adult (>18 years old) | Both | Alzheimer's disease | | details | | Urine | Detected and Quantified | 44.5 +/- 1.3 umol/mmol creatinine | Adult (>18 years old) | Both | Propionic acidemia | | details | | Urine | Detected and Quantified | 57.778 +/- 73.153 umol/mmol creatinine | Children (1 - 13 years old) | Not Specified | Eosinophilic esophagitis | | details |
|
|---|
| Associated Disorders and Diseases |
|---|
| Disease References | | Kidney disease |
|---|
- Raj DS, Ouwendyk M, Francoeur R, Pierratos A: Plasma amino acid profile on nocturnal hemodialysis. Blood Purif. 2000;18(2):97-102. [PubMed:10838467 ]
| | Early preeclampsia |
|---|
- Bahado-Singh RO, Akolekar R, Mandal R, Dong E, Xia J, Kruger M, Wishart DS, Nicolaides K: Metabolomics and first-trimester prediction of early-onset preeclampsia. J Matern Fetal Neonatal Med. 2012 Oct;25(10):1840-7. doi: 10.3109/14767058.2012.680254. Epub 2012 Apr 28. [PubMed:22494326 ]
| | Pregnancy |
|---|
- Bahado-Singh RO, Akolekar R, Mandal R, Dong E, Xia J, Kruger M, Wishart DS, Nicolaides K: Metabolomics and first-trimester prediction of early-onset preeclampsia. J Matern Fetal Neonatal Med. 2012 Oct;25(10):1840-7. doi: 10.3109/14767058.2012.680254. Epub 2012 Apr 28. [PubMed:22494326 ]
| | Alzheimer's disease |
|---|
- Fonteh AN, Harrington RJ, Tsai A, Liao P, Harrington MG: Free amino acid and dipeptide changes in the body fluids from Alzheimer's disease subjects. Amino Acids. 2007 Feb;32(2):213-24. Epub 2006 Oct 10. [PubMed:17031479 ]
| | Colorectal cancer |
|---|
- Goedert JJ, Sampson JN, Moore SC, Xiao Q, Xiong X, Hayes RB, Ahn J, Shi J, Sinha R: Fecal metabolomics: assay performance and association with colorectal cancer. Carcinogenesis. 2014 Sep;35(9):2089-96. doi: 10.1093/carcin/bgu131. Epub 2014 Jul 18. [PubMed:25037050 ]
| | Diabetes mellitus type 2 |
|---|
- Tuma P, Samcova E, Balinova P: Determination of 3-methylhistidine and 1-methylhistidine in untreated urine samples by capillary electrophoresis. J Chromatogr B Analyt Technol Biomed Life Sci. 2005 Jul 5;821(1):53-9. [PubMed:15899597 ]
| | Obesity |
|---|
- Tuma P, Samcova E, Balinova P: Determination of 3-methylhistidine and 1-methylhistidine in untreated urine samples by capillary electrophoresis. J Chromatogr B Analyt Technol Biomed Life Sci. 2005 Jul 5;821(1):53-9. [PubMed:15899597 ]
| | Propionic acidemia |
|---|
- Gronwald W, Klein MS, Kaspar H, Fagerer SR, Nurnberger N, Dettmer K, Bertsch T, Oefner PJ: Urinary metabolite quantification employing 2D NMR spectroscopy. Anal Chem. 2008 Dec 1;80(23):9288-97. doi: 10.1021/ac801627c. [PubMed:19551947 ]
| | Eosinophilic esophagitis |
|---|
- Slae, M., Huynh, H., Wishart, D.S. (2014). Analysis of 30 normal pediatric urine samples via NMR spectroscopy (unpublished work). NA.
|
|
|---|
| Associated OMIM IDs | |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| Phenol Explorer Compound ID | Not Available |
|---|
| FooDB ID | FDB093613 |
|---|
| KNApSAcK ID | Not Available |
|---|
| Chemspider ID | 58494 |
|---|
| KEGG Compound ID | C01152 |
|---|
| BioCyc ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| Wikipedia Link | 3-Methylhistidine |
|---|
| METLIN ID | 5466 |
|---|
| PubChem Compound | 64969 |
|---|
| PDB ID | Not Available |
|---|
| ChEBI ID | 27596 |
|---|
| Food Biomarker Ontology | Not Available |
|---|
| VMH ID | 3MHIS |
|---|
| MarkerDB ID | MDB00000170 |
|---|
| Good Scents ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Ohba, Masashi; Mukaihira, Takafumi; Fujii, Tozo. Preparatory study for the synthesis of the starfish alkaloid imbricatine. Syntheses of 5-arylthio-3-methyl-L-histidines. Chemical & Pharmaceutical Bulletin (1994), 42(9), 1784-90. |
|---|
| Material Safety Data Sheet (MSDS) | Download (PDF) |
|---|
| General References | - Sjolin J, Stjernstrom H, Henneberg S, Hambraeus L, Friman G: Evaluation of urinary 3-methylhistidine excretion in infection by measurements of 1-methylhistidine and the creatinine ratios. Am J Clin Nutr. 1989 Jan;49(1):62-70. [PubMed:2912013 ]
- Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM: Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009 Feb 12;457(7231):910-4. doi: 10.1038/nature07762. [PubMed:19212411 ]
- Bucciante G, Mencini A, Boninsegna A, Branca D, Scutari A, Scutari G: 3-Methylhistidine urinary excretion as an index of skeletal muscle protein metabolism: reference values. G Clin Med. 1985 Nov-Dec;66(11-12):451-8. [PubMed:3835089 ]
- Schmitz JE: [Effect of metabolism-oriented substrate administration on energy and protein metabolism in polytraumatized artificial respiration patients]. Infusionsther Klin Ernahr. 1984 Aug;11(4):205-18. [PubMed:6434422 ]
- McKeran RO, Halliday D, Purkiss P: Comparison of human myofibrillar protein catabolic rate derived from 3-methylhistidine excretion with synthetic rate from muscle biopsies during L-[alpha-15N]lysine infusion. Clin Sci Mol Med. 1978 May;54(5):471-5. [PubMed:750147 ]
- Teahon K, Rideout JM: A sensitive and specific high performance liquid chromatographic assay for imidazole dipeptides and 3-methylhistidine in human muscle biopsies, serum and urine. Biomed Chromatogr. 1992 Jan-Feb;6(1):16-9. [PubMed:1600369 ]
- Adlerberth A, Jagenburg R, Lindstedt G, Stenstrom G, Hasselgren PO: Effects of thyroid hormone and beta-adrenoceptor blocking agents on urinary excretion of 3-methylhistidine and plasma amino acids in man. Eur J Clin Invest. 1986 Aug;16(4):316-20. [PubMed:3093243 ]
- Wang Z, Deurenberg P, Matthews DE, Heymsfield SB: Urinary 3-methylhistidine excretion: association with total body skeletal muscle mass by computerized axial tomography. JPEN J Parenter Enteral Nutr. 1998 Mar-Apr;22(2):82-6. [PubMed:9527964 ]
- Nygren J, Thorell A, Brismar K, Essen P, Wernerman J, McNurlan MA, Garlick PJ, Ljungqvist O: Glucose flux is normalized by compensatory hyperinsulinaemia in growth hormone-induced insulin resistance in healthy subjects, while skeletal muscle protein synthesis remains unchanged. Clin Sci (Lond). 2002 Apr;102(4):457-64. [PubMed:11914108 ]
- Elia M, Carter A, Bacon S, Winearls CG, Smith R: Clinical usefulness of urinary 3-methylhistidine excretion in indicating muscle protein breakdown. Br Med J (Clin Res Ed). 1981 Jan 31;282(6261):351-4. [PubMed:6780020 ]
- Rathmacher JA, Flakoll PJ, Nissen SL: A compartmental model of 3-methylhistidine metabolism in humans. Am J Physiol. 1995 Jul;269(1 Pt 1):E193-8. [PubMed:7631776 ]
- Lunyong VE, Friedman Z: Myofibrillar protein degradation in premature infants with respiratory distress as assessed by 3-methylhistidine and creatinine excretions. Am J Clin Nutr. 1982 Sep;36(3):485-91. [PubMed:7113954 ]
- Buchholz-Wimmer GB, Wimmer W, Herbertz L, Reinauer H: [3-Methylhistidine as a parameter for the determination of muscle proteolysis in the post-stress syndrome and in diabetes mellitus]. Infusionsther Klin Ernahr. 1984 Jun;11(3):168-74. [PubMed:6434416 ]
- Vesali RF, Klaude M, Thunblad L, Rooyackers OE, Wernerman J: Contractile protein breakdown in human leg skeletal muscle as estimated by [2H3]-3-methylhistidine: a new method. Metabolism. 2004 Aug;53(8):1076-80. [PubMed:15281022 ]
- Bird SP, Tarpenning KM, Marino FE: Independent and combined effects of liquid carbohydrate/essential amino acid ingestion on hormonal and muscular adaptations following resistance training in untrained men. Eur J Appl Physiol. 2006 May;97(2):225-38. Epub 2006 Mar 24. [PubMed:16456674 ]
- Lamisse F, May MA, Couet C, Constans T, Bacq Y, Delarue J, Lamagnere JP, Colombat P, Garrigue MA: [Changes in nutritional status at the initial phase of treatment of cancers and malignant hemopathies]. Rev Med Interne. 1987 May-Jun;8(3):257-61. [PubMed:3616232 ]
- Neuhauser M, Bergstrom J, Chao L, Holmstrom J, Nordlund L, Vinnars E, Furst P: Urinary excretion of 3-methylhistidine as an index of muscle protein catabolism in postoperative trauma: the effect of parenteral nutrition. Metabolism. 1980 Dec;29(12):1206-13. [PubMed:6779092 ]
- Emery PW, Rennie MJ: Elimination by formaldehyde of interference with 3-methylhistidine determination: application of the method to the study of muscle protein degradation. Anal Biochem. 1982 Oct;126(1):67-73. [PubMed:7181118 ]
- Tomas FM, Ballard FJ, Pope LM: Age-dependent changes in the rate of myofibrillar protein degradation in humans as assessed by 3-methylhistidine and creatinine excretion. Clin Sci (Lond). 1979 Apr;56(4):341-6. [PubMed:477219 ]
- Long CL, Dillard DR, Bodzin JH, Geiger JW, Blakemore WS: Validity of 3-methylhistidine excretion as an indicator of skeletal muscle protein breakdown in humans. Metabolism. 1988 Sep;37(9):844-9. [PubMed:3138511 ]
- Young VR, Munro HN: Ntau-methylhistidine (3-methylhistidine) and muscle protein turnover: an overview. Fed Proc. 1978 Jul;37(9):2291-300. [PubMed:350635 ]
- Elshenawy S, Pinney SE, Stuart T, Doulias PT, Zura G, Parry S, Elovitz MA, Bennett MJ, Bansal A, Strauss JF 3rd, Ischiropoulos H, Simmons RA: The Metabolomic Signature of the Placenta in Spontaneous Preterm Birth. Int J Mol Sci. 2020 Feb 4;21(3). pii: ijms21031043. doi: 10.3390/ijms21031043. [PubMed:32033212 ]
- Boldyrev AA, Aldini G, Derave W: Physiology and pathophysiology of carnosine. Physiol Rev. 2013 Oct;93(4):1803-45. doi: 10.1152/physrev.00039.2012. [PubMed:24137022 ]
- Chinkes DL: Methods for measuring tissue protein breakdown rate in vivo. Curr Opin Clin Nutr Metab Care. 2005 Sep;8(5):534-7. doi: 10.1097/01.mco.0000170754.25372.37. [PubMed:16079625 ]
- Holecek M: Histidine in Health and Disease: Metabolism, Physiological Importance, and Use as a Supplement. Nutrients. 2020 Mar 22;12(3). pii: nu12030848. doi: 10.3390/nu12030848. [PubMed:32235743 ]
- Said MY, Rodriguez-Nino A, Post A, Schutten JC, Kieneker LM, Gomes-Neto AW, van Londen M, Oste MC, Borgonjen-van den Berg KJ, Nolte IM, van den Berg E, de Blaauw P, van der Krogt J, Heiner-Fokkema MR, Navis G, Yard BA, Bakker SJ: Meat intake and risk of mortality and graft failure in kidney transplant recipients. Am J Clin Nutr. 2021 Jun 5. pii: 6294068. doi: 10.1093/ajcn/nqab185. [PubMed:34091671 ]
|
|---|