| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected and Quantified |
|---|
| Creation Date | 2006-05-22 14:17:43 UTC |
|---|
| Update Date | 2022-03-07 02:49:13 UTC |
|---|
| HMDB ID | HMDB0002226 |
|---|
| Secondary Accession Numbers | - HMDB0062580
- HMDB02226
- HMDB62580
|
|---|
| Metabolite Identification |
|---|
| Common Name | Adrenic acid |
|---|
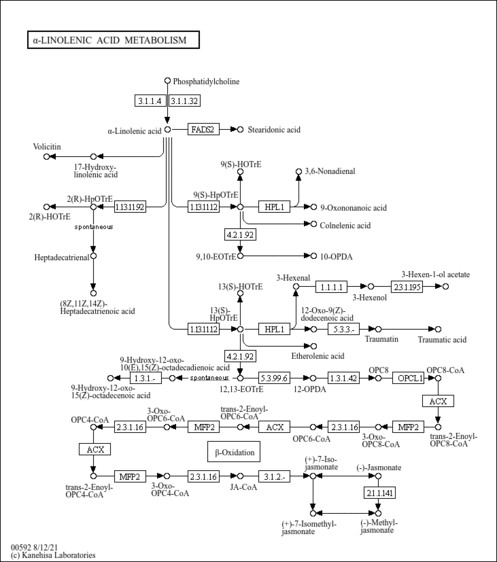
| Description | Adrenic acid, also known as 7,10,13,16-docosatetraenoic acid or adrenate, is a member of the class of compounds known as very long-chain fatty acids. Very long-chain fatty acids are fatty acids with an aliphatic tail that contains at least 22 carbon atoms. Adrenic acid is practically insoluble (in water) and a weakly acidic compound (based on its pKa). Adrenic acid can be found in blood and in human myelin tissue. Within the cell, adrenic acid is primarily located in the cytoplasm, in the membrane (predicted from logP), and in the peroxisome. It can also be found in the extracellular space. In humans, adrenic acid is involved in alpha-linolenic acid and linoleic acid metabolism. Docosatetraenoic acid designates any straight chain 22:4 fatty acid. In particular, all-cis-7,10,13,16-docosatetraenoic acid is an ω-6 fatty acid with the trivial name adrenic acid (AdA). This is a naturally occurring polyunsaturated fatty acid formed through a 2-carbon chain elongation of arachidonic acid. It is one of the most abundant fatty acids in the early human brain. This unsaturated fatty acid is also metabolized by cells into biologically active products, such as dihomoprostaglandins and dihomo-epoxyeicosatrienoic acids (dihomo-EETs) (Wikipedia ). Adrenic acid, which is a prostacyclin inhibitor, appears to be a potential prothrombotic agent (PMID: 1642692 ). |
|---|
| Structure | CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCCCC(O)=O InChI=1S/C22H36O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-21-22(23)24/h6-7,9-10,12-13,15-16H,2-5,8,11,14,17-21H2,1H3,(H,23,24)/b7-6-,10-9-,13-12-,16-15- |
|---|
| Synonyms | | Value | Source |
|---|
| (7Z,10Z,13Z,16Z)-Docosa-7,10,13,16-tetraenoic acid | ChEBI | | 7,10,13,16-Docosatetraenoic acid | ChEBI | | 7Z,10Z,13Z,16Z-Docosatetraenoic acid | ChEBI | | all-cis-7,10,13,16-Docosatetraenoic acid | ChEBI | | cis,cis,cis,cis-Docosa-7,10,13,16-tetraensaeure | ChEBI | | (7Z,10Z,13Z,16Z)-Docosa-7,10,13,16-tetraenoate | Generator | | 7,10,13,16-Docosatetraenoate | Generator | | 7Z,10Z,13Z,16Z-Docosatetraenoate | Generator | | all-cis-7,10,13,16-Docosatetraenoate | Generator | | Adrenate | Generator | | Adrenic acid, (Z)-isomer | HMDB | | 7,10,13,16-Docosatetraenoic acid (van) adrenate | HMDB | | 7,10,13,16-Docosatetraenoic acid (van) adrenic acid | HMDB | | FA(22:4(7Z,10Z,13Z,16Z)) | HMDB | | (7Z,10Z,13Z,16Z)-Docosatetraenoate | HMDB |
|
|---|
| Chemical Formula | C22H36O2 |
|---|
| Average Molecular Weight | 332.52 |
|---|
| Monoisotopic Molecular Weight | 332.271530396 |
|---|
| IUPAC Name | (7Z,10Z,13Z,16Z)-docosa-7,10,13,16-tetraenoic acid |
|---|
| Traditional Name | docosatetraenoic acid |
|---|
| CAS Registry Number | 28874-58-0 |
|---|
| SMILES | CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCCCC(O)=O |
|---|
| InChI Identifier | InChI=1S/C22H36O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-21-22(23)24/h6-7,9-10,12-13,15-16H,2-5,8,11,14,17-21H2,1H3,(H,23,24)/b7-6-,10-9-,13-12-,16-15- |
|---|
| InChI Key | TWSWSIQAPQLDBP-DOFZRALJSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as very long-chain fatty acids. These are fatty acids with an aliphatic tail that contains at least 22 carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acids and conjugates |
|---|
| Direct Parent | Very long-chain fatty acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Very long-chain fatty acid
- Unsaturated fatty acid
- Straight chain fatty acid
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | Not Available |
|---|
| Role | Not Available |
|---|
| Physical Properties |
|---|
| State | Liquid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Experimental Collision Cross Sections |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Retention Times Underivatized| Chromatographic Method | Retention Time | Reference |
|---|
| Measured using a Waters Acquity ultraperformance liquid chromatography (UPLC) ethylene-bridged hybrid (BEH) C18 column (100 mm × 2.1 mm; 1.7 μmparticle diameter). Predicted by Afia on May 17, 2022. Predicted by Afia on May 17, 2022. | 7.96 minutes | 32390414 | | Predicted by Siyang on May 30, 2022 | 29.1109 minutes | 33406817 | | Predicted by Siyang using ReTip algorithm on June 8, 2022 | 1.6 minutes | 32390414 | | AjsUoB = Accucore 150 Amide HILIC with 10mM Ammonium Formate, 0.1% Formic Acid | 41.2 seconds | 40023050 | | Fem_Long = Waters ACQUITY UPLC HSS T3 C18 with Water:MeOH and 0.1% Formic Acid | 3475.9 seconds | 40023050 | | Fem_Lipids = Ascentis Express C18 with (60:40 water:ACN):(90:10 IPA:ACN) and 10mM NH4COOH + 0.1% Formic Acid | 727.9 seconds | 40023050 | | Life_Old = Waters ACQUITY UPLC BEH C18 with Water:(20:80 acetone:ACN) and 0.1% Formic Acid | 296.4 seconds | 40023050 | | Life_New = RP Waters ACQUITY UPLC HSS T3 C18 with Water:(30:70 MeOH:ACN) and 0.1% Formic Acid | 535.6 seconds | 40023050 | | RIKEN = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 813.5 seconds | 40023050 | | Eawag_XBridgeC18 = XBridge C18 3.5u 2.1x50 mm with Water:MeOH and 0.1% Formic Acid | 1372.4 seconds | 40023050 | | BfG_NTS_RP1 =Agilent Zorbax Eclipse Plus C18 (2.1 mm x 150 mm, 3.5 um) with Water:ACN and 0.1% Formic Acid | 783.4 seconds | 40023050 | | HILIC_BDD_2 = Merck SeQuant ZIC-HILIC with ACN(0.1% formic acid):water(16 mM ammonium formate) | 97.1 seconds | 40023050 | | UniToyama_Atlantis = RP Waters Atlantis T3 (2.1 x 150 mm, 5 um) with ACN:Water and 0.1% Formic Acid | 2709.5 seconds | 40023050 | | BDD_C18 = Hypersil Gold 1.9µm C18 with Water:ACN and 0.1% Formic Acid | 885.6 seconds | 40023050 | | UFZ_Phenomenex = Kinetex Core-Shell C18 2.6 um, 3.0 x 100 mm, Phenomenex with Water:MeOH and 0.1% Formic Acid | 2196.4 seconds | 40023050 | | SNU_RIKEN_POS = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 1028.1 seconds | 40023050 | | RPMMFDA = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 611.5 seconds | 40023050 | | MTBLS87 = Merck SeQuant ZIC-pHILIC column with ACN:Water and :ammonium carbonate | 603.9 seconds | 40023050 | | KI_GIAR_zic_HILIC_pH2_7 = Merck SeQuant ZIC-HILIC with ACN:Water and 0.1% FA | 705.5 seconds | 40023050 | | Meister zic-pHILIC pH9.3 = Merck SeQuant ZIC-pHILIC column with ACN:Water 5mM NH4Ac pH9.3 and 5mM ammonium acetate in water | 9.0 seconds | 40023050 |
Predicted Kovats Retention IndicesUnderivatizedDerivatized |
|---|
| General References | - Martinez M, Mougan I: Fatty acid composition of brain glycerophospholipids in peroxisomal disorders. Lipids. 1999 Jul;34(7):733-40. [PubMed:10478932 ]
- Ren H, Magulike N, Ghebremeskel K, Crawford M: Primary open-angle glaucoma patients have reduced levels of blood docosahexaenoic and eicosapentaenoic acids. Prostaglandins Leukot Essent Fatty Acids. 2006 Mar;74(3):157-63. Epub 2006 Jan 10. [PubMed:16410047 ]
- Tardy B, Bordet JC, Berruyer M, Ffrench P, Dechavanne M: Priming effect of adrenic acid (22:4(n-6)) on tissue factor activity expressed by thrombin-stimulated endothelial cells. Atherosclerosis. 1992 Jul;95(1):51-8. [PubMed:1642692 ]
- Simons K, Toomre D: Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000 Oct;1(1):31-9. [PubMed:11413487 ]
- Watson AD: Thematic review series: systems biology approaches to metabolic and cardiovascular disorders. Lipidomics: a global approach to lipid analysis in biological systems. J Lipid Res. 2006 Oct;47(10):2101-11. Epub 2006 Aug 10. [PubMed:16902246 ]
- Sethi JK, Vidal-Puig AJ: Thematic review series: adipocyte biology. Adipose tissue function and plasticity orchestrate nutritional adaptation. J Lipid Res. 2007 Jun;48(6):1253-62. Epub 2007 Mar 20. [PubMed:17374880 ]
- Lingwood D, Simons K: Lipid rafts as a membrane-organizing principle. Science. 2010 Jan 1;327(5961):46-50. doi: 10.1126/science.1174621. [PubMed:20044567 ]
- Elshenawy S, Pinney SE, Stuart T, Doulias PT, Zura G, Parry S, Elovitz MA, Bennett MJ, Bansal A, Strauss JF 3rd, Ischiropoulos H, Simmons RA: The Metabolomic Signature of the Placenta in Spontaneous Preterm Birth. Int J Mol Sci. 2020 Feb 4;21(3). pii: ijms21031043. doi: 10.3390/ijms21031043. [PubMed:32033212 ]
- Gunstone, Frank D., John L. Harwood, and Albert J. Dijkstra (2007). The lipid handbook with CD-ROM. CRC Press.
|
|---|