| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected and Quantified |
|---|
| Creation Date | 2006-08-12 23:23:28 UTC |
|---|
| Update Date | 2021-09-14 15:47:19 UTC |
|---|
| HMDB ID | HMDB0003540 |
|---|
| Secondary Accession Numbers | - HMDB0006550
- HMDB03540
- HMDB06550
|
|---|
| Metabolite Identification |
|---|
| Common Name | 3'-AMP |
|---|
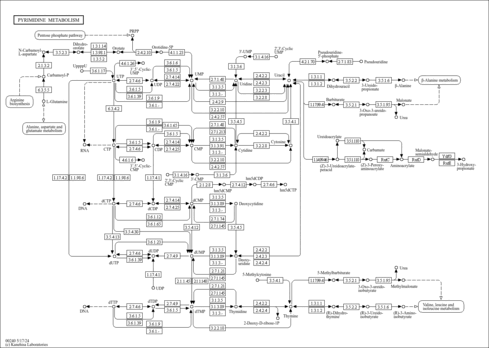
| Description | 3'-AMP, also known as 3'-adenylic acid or AMP 3'-phosphate, belongs to the class of organic compounds known as ribonucleoside 3'-phosphates. These are ribonucleosides that contain a phosphate group attached to the C-3 carbon of the ribose or deoxyribose moiety. The nucleobases here are limited to purine, pyrimidine, and pyridine derivatives. 3'-AMP exists in all living organisms, ranging from bacteria to humans. In humans, 3'-AMP is involved in the pyrimidine metabolism pathway. 3'-AMP has been detected, but not quantified in, a few different foods, such as anatidaes (Anatidae), chickens (Gallus gallus), and domestic pigs (Sus scrofa domestica). This could make 3'-AMP a potential biomarker for the consumption of these foods. 3'-AMP is a secondary metabolite. Secondary metabolites are metabolically or physiologically non-essential metabolites that may serve a role as defense or signalling molecules. In some cases they are simply molecules that arise from the incomplete metabolism of other secondary metabolites. Based on a literature review a small amount of articles have been published on 3'-AMP. |
|---|
| Structure | NC1=C2N=CN([C@@H]3O[C@H](CO)[C@@H](OP(O)(O)=O)[C@H]3O)C2=NC=N1 InChI=1S/C10H14N5O7P/c11-8-5-9(13-2-12-8)15(3-14-5)10-6(17)7(4(1-16)21-10)22-23(18,19)20/h2-4,6-7,10,16-17H,1H2,(H2,11,12,13)(H2,18,19,20)/t4-,6-,7-,10-/m1/s1 |
|---|
| Synonyms | | Value | Source |
|---|
| 3'-Adenosine monophosphate | ChEBI | | 3'-Adenylic acid | ChEBI | | Adenosine 3'-monophosphate | ChEBI | | Adenosine 3'-phosphate | ChEBI | | Adenosine-3'-monophosphate | ChEBI | | AMP 3'-Phosphate | ChEBI | | Synadenylic acid | ChEBI | | 3'-Adenosine monophosphoric acid | Generator | | 3'-Adenylate | Generator | | Adenosine 3'-monophosphoric acid | Generator | | Adenosine 3'-phosphoric acid | Generator | | Adenosine-3'-monophosphoric acid | Generator | | AMP 3'-Phosphoric acid | Generator | | Synadenylate | Generator | | Adenosine-3'-phosphate | HMDB | | Yeast adenylic acid | HMDB | | 2' Adenylic acid | MeSH, HMDB | | 5' Adenylic acid | MeSH, HMDB | | Phosphate dipotassium, adenosine | MeSH, HMDB | | 5'-Adenylic acid | MeSH, HMDB | | Adenylic acid | MeSH, HMDB | | 5'-Phosphate, adenosine | MeSH, HMDB | | Adenosine 2'-phosphate | MeSH, HMDB | | Dipotassium, adenosine phosphate | MeSH, HMDB | | Phosphate disodium, adenosine | MeSH, HMDB | | Monophosphate, 2'-adenosine | MeSH, HMDB | | 2'-AMP | MeSH, HMDB | | 2'-Adenosine monophosphate | MeSH, HMDB | | Adenosine 2' phosphate | MeSH, HMDB | | Adenosine phosphate dipotassium | MeSH, HMDB | | Adenosine 3' phosphate | MeSH, HMDB | | Disodium, adenosine phosphate | MeSH, HMDB | | Phosphaden | MeSH, HMDB | | 2'-Adenylic acid | MeSH, HMDB | | AMP | MeSH, HMDB | | Adenosine monophosphate | MeSH, HMDB | | Adenosine phosphate disodium | MeSH, HMDB | | 2' Adenosine monophosphate | MeSH, HMDB | | Acid, 2'-adenylic | MeSH, HMDB | | Acid, 5'-adenylic | MeSH, HMDB | | Adenosine 5' phosphate | MeSH, HMDB | | Adenosine 5'-phosphate | MeSH, HMDB |
|
|---|
| Chemical Formula | C10H14N5O7P |
|---|
| Average Molecular Weight | 347.2212 |
|---|
| Monoisotopic Molecular Weight | 347.063084339 |
|---|
| IUPAC Name | {[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-2-(hydroxymethyl)oxolan-3-yl]oxy}phosphonic acid |
|---|
| Traditional Name | adenosine-3'-phosphate |
|---|
| CAS Registry Number | 84-21-9 |
|---|
| SMILES | NC1=C2N=CN([C@@H]3O[C@H](CO)[C@@H](OP(O)(O)=O)[C@H]3O)C2=NC=N1 |
|---|
| InChI Identifier | InChI=1S/C10H14N5O7P/c11-8-5-9(13-2-12-8)15(3-14-5)10-6(17)7(4(1-16)21-10)22-23(18,19)20/h2-4,6-7,10,16-17H,1H2,(H2,11,12,13)(H2,18,19,20)/t4-,6-,7-,10-/m1/s1 |
|---|
| InChI Key | LNQVTSROQXJCDD-KQYNXXCUSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as ribonucleoside 3'-phosphates. These are ribonucleosides that contain a phosphate group attached to the C-3 carbon of the ribose or deoxyribose moiety. The nucleobases here are limited to purine, pyrimidine, and pyridine derivatives. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Nucleosides, nucleotides, and analogues |
|---|
| Class | Ribonucleoside 3'-phosphates |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Ribonucleoside 3'-phosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pentose phosphate
- Ribonucleoside 3'-phosphate
- Glycosyl compound
- N-glycosyl compound
- 6-aminopurine
- Pentose monosaccharide
- Monosaccharide phosphate
- Purine
- Imidazopyrimidine
- Aminopyrimidine
- Monoalkyl phosphate
- Organic phosphoric acid derivative
- Phosphoric acid ester
- N-substituted imidazole
- Monosaccharide
- Pyrimidine
- Imidolactam
- Alkyl phosphate
- Heteroaromatic compound
- Tetrahydrofuran
- Azole
- Imidazole
- Secondary alcohol
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Primary amine
- Amine
- Alcohol
- Organic nitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Primary alcohol
- Organooxygen compound
- Organonitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | 197 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 500 mg/L at 15 °C | Not Available | | LogP | -1.453 | Not Available |
|
|---|
| Experimental Chromatographic Properties | Experimental Collision Cross Sections |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Retention Times Underivatized| Chromatographic Method | Retention Time | Reference |
|---|
| Measured using a Waters Acquity ultraperformance liquid chromatography (UPLC) ethylene-bridged hybrid (BEH) C18 column (100 mm × 2.1 mm; 1.7 μmparticle diameter). Predicted by Afia on May 17, 2022. Predicted by Afia on May 17, 2022. | 2.64 minutes | 32390414 | | Measured using a Waters Acquity ultraperformance liquid chromatography (UPLC) ethylene-bridged hybrid (BEH) C18 column (100 mm × 2.1 mm; 1.7 μmparticle diameter). Predicted by Afia on May 17, 2022. Predicted by Afia on May 17, 2022. | 2.44 minutes | 32390414 | | Predicted by Siyang on May 30, 2022 | 9.1789 minutes | 33406817 | | Predicted by Siyang using ReTip algorithm on June 8, 2022 | 8.94 minutes | 32390414 | | AjsUoB = Accucore 150 Amide HILIC with 10mM Ammonium Formate, 0.1% Formic Acid | 397.4 seconds | 40023050 | | Fem_Long = Waters ACQUITY UPLC HSS T3 C18 with Water:MeOH and 0.1% Formic Acid | 728.0 seconds | 40023050 | | Fem_Lipids = Ascentis Express C18 with (60:40 water:ACN):(90:10 IPA:ACN) and 10mM NH4COOH + 0.1% Formic Acid | 228.9 seconds | 40023050 | | Life_Old = Waters ACQUITY UPLC BEH C18 with Water:(20:80 acetone:ACN) and 0.1% Formic Acid | 44.9 seconds | 40023050 | | Life_New = RP Waters ACQUITY UPLC HSS T3 C18 with Water:(30:70 MeOH:ACN) and 0.1% Formic Acid | 153.3 seconds | 40023050 | | RIKEN = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 60.8 seconds | 40023050 | | Eawag_XBridgeC18 = XBridge C18 3.5u 2.1x50 mm with Water:MeOH and 0.1% Formic Acid | 346.1 seconds | 40023050 | | BfG_NTS_RP1 =Agilent Zorbax Eclipse Plus C18 (2.1 mm x 150 mm, 3.5 um) with Water:ACN and 0.1% Formic Acid | 264.3 seconds | 40023050 | | HILIC_BDD_2 = Merck SeQuant ZIC-HILIC with ACN(0.1% formic acid):water(16 mM ammonium formate) | 829.4 seconds | 40023050 | | UniToyama_Atlantis = RP Waters Atlantis T3 (2.1 x 150 mm, 5 um) with ACN:Water and 0.1% Formic Acid | 553.3 seconds | 40023050 | | BDD_C18 = Hypersil Gold 1.9µm C18 with Water:ACN and 0.1% Formic Acid | 43.5 seconds | 40023050 | | UFZ_Phenomenex = Kinetex Core-Shell C18 2.6 um, 3.0 x 100 mm, Phenomenex with Water:MeOH and 0.1% Formic Acid | 594.2 seconds | 40023050 | | SNU_RIKEN_POS = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 157.3 seconds | 40023050 | | RPMMFDA = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 177.4 seconds | 40023050 | | MTBLS87 = Merck SeQuant ZIC-pHILIC column with ACN:Water and :ammonium carbonate | 673.3 seconds | 40023050 | | KI_GIAR_zic_HILIC_pH2_7 = Merck SeQuant ZIC-HILIC with ACN:Water and 0.1% FA | 477.5 seconds | 40023050 | | Meister zic-pHILIC pH9.3 = Merck SeQuant ZIC-pHILIC column with ACN:Water 5mM NH4Ac pH9.3 and 5mM ammonium acetate in water | 358.8 seconds | 40023050 |
Predicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| 3'-AMP,1TMS,isomer #1 | C[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@H](O)[C@@H]1OP(=O)(O)O | 3111.3 | Semi standard non polar | 33892256 | | 3'-AMP,1TMS,isomer #2 | C[Si](C)(C)O[C@@H]1[C@H](OP(=O)(O)O)[C@@H](CO)O[C@H]1N1C=NC2=C(N)N=CN=C21 | 3085.2 | Semi standard non polar | 33892256 | | 3'-AMP,1TMS,isomer #3 | C[Si](C)(C)OP(=O)(O)O[C@@H]1[C@@H](CO)O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@@H]1O | 3131.9 | Semi standard non polar | 33892256 | | 3'-AMP,1TMS,isomer #4 | C[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO)[C@@H](OP(=O)(O)O)[C@H]1O | 3135.7 | Semi standard non polar | 33892256 | | 3'-AMP,2TMS,isomer #1 | C[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1OP(=O)(O)O | 3006.2 | Semi standard non polar | 33892256 | | 3'-AMP,2TMS,isomer #2 | C[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@H](O)[C@@H]1OP(=O)(O)O[Si](C)(C)C | 3055.1 | Semi standard non polar | 33892256 | | 3'-AMP,2TMS,isomer #3 | C[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO[Si](C)(C)C)[C@@H](OP(=O)(O)O)[C@H]1O | 3058.6 | Semi standard non polar | 33892256 | | 3'-AMP,2TMS,isomer #4 | C[Si](C)(C)O[C@@H]1[C@H](OP(=O)(O)O[Si](C)(C)C)[C@@H](CO)O[C@H]1N1C=NC2=C(N)N=CN=C21 | 3037.4 | Semi standard non polar | 33892256 | | 3'-AMP,2TMS,isomer #5 | C[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO)[C@@H](OP(=O)(O)O)[C@H]1O[Si](C)(C)C | 3041.9 | Semi standard non polar | 33892256 | | 3'-AMP,2TMS,isomer #6 | C[Si](C)(C)OP(=O)(O[C@@H]1[C@@H](CO)O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@@H]1O)O[Si](C)(C)C | 3063.5 | Semi standard non polar | 33892256 | | 3'-AMP,2TMS,isomer #7 | C[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO)[C@@H](OP(=O)(O)O[Si](C)(C)C)[C@H]1O | 3104.3 | Semi standard non polar | 33892256 | | 3'-AMP,2TMS,isomer #8 | C[Si](C)(C)N(C1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO)[C@@H](OP(=O)(O)O)[C@H]1O)[Si](C)(C)C | 3114.7 | Semi standard non polar | 33892256 | | 3'-AMP,3TMS,isomer #1 | C[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1OP(=O)(O)O[Si](C)(C)C | 2989.4 | Semi standard non polar | 33892256 | | 3'-AMP,3TMS,isomer #1 | C[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1OP(=O)(O)O[Si](C)(C)C | 2985.4 | Standard non polar | 33892256 | | 3'-AMP,3TMS,isomer #1 | C[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1OP(=O)(O)O[Si](C)(C)C | 4830.7 | Standard polar | 33892256 | | 3'-AMP,3TMS,isomer #10 | C[Si](C)(C)OP(=O)(O)O[C@@H]1[C@@H](CO)O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@@H]1O | 3082.9 | Semi standard non polar | 33892256 | | 3'-AMP,3TMS,isomer #10 | C[Si](C)(C)OP(=O)(O)O[C@@H]1[C@@H](CO)O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@@H]1O | 3127.9 | Standard non polar | 33892256 | | 3'-AMP,3TMS,isomer #10 | C[Si](C)(C)OP(=O)(O)O[C@@H]1[C@@H](CO)O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@@H]1O | 4421.3 | Standard polar | 33892256 | | 3'-AMP,3TMS,isomer #2 | C[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO[Si](C)(C)C)[C@@H](OP(=O)(O)O)[C@H]1O[Si](C)(C)C | 3000.3 | Semi standard non polar | 33892256 | | 3'-AMP,3TMS,isomer #2 | C[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO[Si](C)(C)C)[C@@H](OP(=O)(O)O)[C@H]1O[Si](C)(C)C | 3066.4 | Standard non polar | 33892256 | | 3'-AMP,3TMS,isomer #2 | C[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO[Si](C)(C)C)[C@@H](OP(=O)(O)O)[C@H]1O[Si](C)(C)C | 4937.3 | Standard polar | 33892256 | | 3'-AMP,3TMS,isomer #3 | C[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@H](O)[C@@H]1OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 3016.8 | Semi standard non polar | 33892256 | | 3'-AMP,3TMS,isomer #3 | C[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@H](O)[C@@H]1OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 3031.5 | Standard non polar | 33892256 | | 3'-AMP,3TMS,isomer #3 | C[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@H](O)[C@@H]1OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 4672.2 | Standard polar | 33892256 | | 3'-AMP,3TMS,isomer #4 | C[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO[Si](C)(C)C)[C@@H](OP(=O)(O)O[Si](C)(C)C)[C@H]1O | 3043.0 | Semi standard non polar | 33892256 | | 3'-AMP,3TMS,isomer #4 | C[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO[Si](C)(C)C)[C@@H](OP(=O)(O)O[Si](C)(C)C)[C@H]1O | 3085.9 | Standard non polar | 33892256 | | 3'-AMP,3TMS,isomer #4 | C[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO[Si](C)(C)C)[C@@H](OP(=O)(O)O[Si](C)(C)C)[C@H]1O | 4685.0 | Standard polar | 33892256 | | 3'-AMP,3TMS,isomer #5 | C[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@H](O)[C@@H]1OP(=O)(O)O | 3025.9 | Semi standard non polar | 33892256 | | 3'-AMP,3TMS,isomer #5 | C[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@H](O)[C@@H]1OP(=O)(O)O | 3168.9 | Standard non polar | 33892256 | | 3'-AMP,3TMS,isomer #5 | C[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@H](O)[C@@H]1OP(=O)(O)O | 4724.8 | Standard polar | 33892256 | | 3'-AMP,3TMS,isomer #6 | C[Si](C)(C)O[C@@H]1[C@H](OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)[C@@H](CO)O[C@H]1N1C=NC2=C(N)N=CN=C21 | 3010.0 | Semi standard non polar | 33892256 | | 3'-AMP,3TMS,isomer #6 | C[Si](C)(C)O[C@@H]1[C@H](OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)[C@@H](CO)O[C@H]1N1C=NC2=C(N)N=CN=C21 | 3006.7 | Standard non polar | 33892256 | | 3'-AMP,3TMS,isomer #6 | C[Si](C)(C)O[C@@H]1[C@H](OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)[C@@H](CO)O[C@H]1N1C=NC2=C(N)N=CN=C21 | 4660.8 | Standard polar | 33892256 | | 3'-AMP,3TMS,isomer #7 | C[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO)[C@@H](OP(=O)(O)O[Si](C)(C)C)[C@H]1O[Si](C)(C)C | 3036.5 | Semi standard non polar | 33892256 | | 3'-AMP,3TMS,isomer #7 | C[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO)[C@@H](OP(=O)(O)O[Si](C)(C)C)[C@H]1O[Si](C)(C)C | 3086.6 | Standard non polar | 33892256 | | 3'-AMP,3TMS,isomer #7 | C[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO)[C@@H](OP(=O)(O)O[Si](C)(C)C)[C@H]1O[Si](C)(C)C | 4657.9 | Standard polar | 33892256 | | 3'-AMP,3TMS,isomer #8 | C[Si](C)(C)O[C@@H]1[C@H](OP(=O)(O)O)[C@@H](CO)O[C@H]1N1C=NC2=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C21 | 3034.8 | Semi standard non polar | 33892256 | | 3'-AMP,3TMS,isomer #8 | C[Si](C)(C)O[C@@H]1[C@H](OP(=O)(O)O)[C@@H](CO)O[C@H]1N1C=NC2=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C21 | 3150.3 | Standard non polar | 33892256 | | 3'-AMP,3TMS,isomer #8 | C[Si](C)(C)O[C@@H]1[C@H](OP(=O)(O)O)[C@@H](CO)O[C@H]1N1C=NC2=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C21 | 4692.1 | Standard polar | 33892256 | | 3'-AMP,3TMS,isomer #9 | C[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO)[C@@H](OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)[C@H]1O | 3063.0 | Semi standard non polar | 33892256 | | 3'-AMP,3TMS,isomer #9 | C[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO)[C@@H](OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)[C@H]1O | 3123.0 | Standard non polar | 33892256 | | 3'-AMP,3TMS,isomer #9 | C[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO)[C@@H](OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)[C@H]1O | 4446.4 | Standard polar | 33892256 | | 3'-AMP,4TMS,isomer #1 | C[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 3007.4 | Semi standard non polar | 33892256 | | 3'-AMP,4TMS,isomer #1 | C[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 2971.4 | Standard non polar | 33892256 | | 3'-AMP,4TMS,isomer #1 | C[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 4531.1 | Standard polar | 33892256 | | 3'-AMP,4TMS,isomer #2 | C[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO[Si](C)(C)C)[C@@H](OP(=O)(O)O[Si](C)(C)C)[C@H]1O[Si](C)(C)C | 3029.7 | Semi standard non polar | 33892256 | | 3'-AMP,4TMS,isomer #2 | C[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO[Si](C)(C)C)[C@@H](OP(=O)(O)O[Si](C)(C)C)[C@H]1O[Si](C)(C)C | 3050.8 | Standard non polar | 33892256 | | 3'-AMP,4TMS,isomer #2 | C[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO[Si](C)(C)C)[C@@H](OP(=O)(O)O[Si](C)(C)C)[C@H]1O[Si](C)(C)C | 4431.0 | Standard polar | 33892256 | | 3'-AMP,4TMS,isomer #3 | C[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1OP(=O)(O)O | 3008.0 | Semi standard non polar | 33892256 | | 3'-AMP,4TMS,isomer #3 | C[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1OP(=O)(O)O | 3128.8 | Standard non polar | 33892256 | | 3'-AMP,4TMS,isomer #3 | C[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1OP(=O)(O)O | 4385.9 | Standard polar | 33892256 | | 3'-AMP,4TMS,isomer #4 | C[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO[Si](C)(C)C)[C@@H](OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)[C@H]1O | 3053.2 | Semi standard non polar | 33892256 | | 3'-AMP,4TMS,isomer #4 | C[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO[Si](C)(C)C)[C@@H](OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)[C@H]1O | 3066.8 | Standard non polar | 33892256 | | 3'-AMP,4TMS,isomer #4 | C[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO[Si](C)(C)C)[C@@H](OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)[C@H]1O | 4243.0 | Standard polar | 33892256 | | 3'-AMP,4TMS,isomer #5 | C[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@H](O)[C@@H]1OP(=O)(O)O[Si](C)(C)C | 3047.0 | Semi standard non polar | 33892256 | | 3'-AMP,4TMS,isomer #5 | C[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@H](O)[C@@H]1OP(=O)(O)O[Si](C)(C)C | 3107.6 | Standard non polar | 33892256 | | 3'-AMP,4TMS,isomer #5 | C[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@H](O)[C@@H]1OP(=O)(O)O[Si](C)(C)C | 4186.4 | Standard polar | 33892256 | | 3'-AMP,4TMS,isomer #6 | C[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO)[C@@H](OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)[C@H]1O[Si](C)(C)C | 3052.5 | Semi standard non polar | 33892256 | | 3'-AMP,4TMS,isomer #6 | C[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO)[C@@H](OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)[C@H]1O[Si](C)(C)C | 3076.5 | Standard non polar | 33892256 | | 3'-AMP,4TMS,isomer #6 | C[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO)[C@@H](OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)[C@H]1O[Si](C)(C)C | 4223.3 | Standard polar | 33892256 | | 3'-AMP,4TMS,isomer #7 | C[Si](C)(C)O[C@@H]1[C@H](OP(=O)(O)O[Si](C)(C)C)[C@@H](CO)O[C@H]1N1C=NC2=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C21 | 3053.4 | Semi standard non polar | 33892256 | | 3'-AMP,4TMS,isomer #7 | C[Si](C)(C)O[C@@H]1[C@H](OP(=O)(O)O[Si](C)(C)C)[C@@H](CO)O[C@H]1N1C=NC2=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C21 | 3108.4 | Standard non polar | 33892256 | | 3'-AMP,4TMS,isomer #7 | C[Si](C)(C)O[C@@H]1[C@H](OP(=O)(O)O[Si](C)(C)C)[C@@H](CO)O[C@H]1N1C=NC2=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C21 | 4165.0 | Standard polar | 33892256 | | 3'-AMP,4TMS,isomer #8 | C[Si](C)(C)OP(=O)(O[C@@H]1[C@@H](CO)O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@@H]1O)O[Si](C)(C)C | 3078.6 | Semi standard non polar | 33892256 | | 3'-AMP,4TMS,isomer #8 | C[Si](C)(C)OP(=O)(O[C@@H]1[C@@H](CO)O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@@H]1O)O[Si](C)(C)C | 3121.7 | Standard non polar | 33892256 | | 3'-AMP,4TMS,isomer #8 | C[Si](C)(C)OP(=O)(O[C@@H]1[C@@H](CO)O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@@H]1O)O[Si](C)(C)C | 3962.8 | Standard polar | 33892256 | | 3'-AMP,5TMS,isomer #1 | C[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO[Si](C)(C)C)[C@@H](OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)[C@H]1O[Si](C)(C)C | 3069.0 | Semi standard non polar | 33892256 | | 3'-AMP,5TMS,isomer #1 | C[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO[Si](C)(C)C)[C@@H](OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)[C@H]1O[Si](C)(C)C | 3023.2 | Standard non polar | 33892256 | | 3'-AMP,5TMS,isomer #1 | C[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO[Si](C)(C)C)[C@@H](OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)[C@H]1O[Si](C)(C)C | 4045.1 | Standard polar | 33892256 | | 3'-AMP,5TMS,isomer #2 | C[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1OP(=O)(O)O[Si](C)(C)C | 3062.2 | Semi standard non polar | 33892256 | | 3'-AMP,5TMS,isomer #2 | C[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1OP(=O)(O)O[Si](C)(C)C | 3047.8 | Standard non polar | 33892256 | | 3'-AMP,5TMS,isomer #2 | C[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1OP(=O)(O)O[Si](C)(C)C | 3919.6 | Standard polar | 33892256 | | 3'-AMP,5TMS,isomer #3 | C[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@H](O)[C@@H]1OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 3083.8 | Semi standard non polar | 33892256 | | 3'-AMP,5TMS,isomer #3 | C[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@H](O)[C@@H]1OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 3054.5 | Standard non polar | 33892256 | | 3'-AMP,5TMS,isomer #3 | C[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@H](O)[C@@H]1OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 3779.3 | Standard polar | 33892256 | | 3'-AMP,5TMS,isomer #4 | C[Si](C)(C)O[C@@H]1[C@H](OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)[C@@H](CO)O[C@H]1N1C=NC2=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C21 | 3087.2 | Semi standard non polar | 33892256 | | 3'-AMP,5TMS,isomer #4 | C[Si](C)(C)O[C@@H]1[C@H](OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)[C@@H](CO)O[C@H]1N1C=NC2=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C21 | 3063.1 | Standard non polar | 33892256 | | 3'-AMP,5TMS,isomer #4 | C[Si](C)(C)O[C@@H]1[C@H](OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)[C@@H](CO)O[C@H]1N1C=NC2=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C21 | 3759.0 | Standard polar | 33892256 | | 3'-AMP,6TMS,isomer #1 | C[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 3114.5 | Semi standard non polar | 33892256 | | 3'-AMP,6TMS,isomer #1 | C[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 2993.4 | Standard non polar | 33892256 | | 3'-AMP,6TMS,isomer #1 | C[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 3626.6 | Standard polar | 33892256 | | 3'-AMP,1TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@H](O)[C@@H]1OP(=O)(O)O | 3361.7 | Semi standard non polar | 33892256 | | 3'-AMP,1TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@H](OP(=O)(O)O)[C@@H](CO)O[C@H]1N1C=NC2=C(N)N=CN=C21 | 3339.5 | Semi standard non polar | 33892256 | | 3'-AMP,1TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OP(=O)(O)O[C@@H]1[C@@H](CO)O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@@H]1O | 3357.8 | Semi standard non polar | 33892256 | | 3'-AMP,1TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO)[C@@H](OP(=O)(O)O)[C@H]1O | 3359.1 | Semi standard non polar | 33892256 | | 3'-AMP,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1OP(=O)(O)O | 3485.1 | Semi standard non polar | 33892256 | | 3'-AMP,2TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@H](O)[C@@H]1OP(=O)(O)O[Si](C)(C)C(C)(C)C | 3501.7 | Semi standard non polar | 33892256 | | 3'-AMP,2TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO[Si](C)(C)C(C)(C)C)[C@@H](OP(=O)(O)O)[C@H]1O | 3464.7 | Semi standard non polar | 33892256 | | 3'-AMP,2TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@H](OP(=O)(O)O[Si](C)(C)C(C)(C)C)[C@@H](CO)O[C@H]1N1C=NC2=C(N)N=CN=C21 | 3478.7 | Semi standard non polar | 33892256 | | 3'-AMP,2TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO)[C@@H](OP(=O)(O)O)[C@H]1O[Si](C)(C)C(C)(C)C | 3448.6 | Semi standard non polar | 33892256 | | 3'-AMP,2TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)OP(=O)(O[C@@H]1[C@@H](CO)O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@@H]1O)O[Si](C)(C)C(C)(C)C | 3493.4 | Semi standard non polar | 33892256 | | 3'-AMP,2TBDMS,isomer #7 | CC(C)(C)[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO)[C@@H](OP(=O)(O)O[Si](C)(C)C(C)(C)C)[C@H]1O | 3492.6 | Semi standard non polar | 33892256 | | 3'-AMP,2TBDMS,isomer #8 | CC(C)(C)[Si](C)(C)N(C1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO)[C@@H](OP(=O)(O)O)[C@H]1O)[Si](C)(C)C(C)(C)C | 3513.0 | Semi standard non polar | 33892256 | | 3'-AMP,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1OP(=O)(O)O[Si](C)(C)C(C)(C)C | 3594.5 | Semi standard non polar | 33892256 | | 3'-AMP,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1OP(=O)(O)O[Si](C)(C)C(C)(C)C | 3529.2 | Standard non polar | 33892256 | | 3'-AMP,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1OP(=O)(O)O[Si](C)(C)C(C)(C)C | 4925.9 | Standard polar | 33892256 | | 3'-AMP,3TBDMS,isomer #10 | CC(C)(C)[Si](C)(C)OP(=O)(O)O[C@@H]1[C@@H](CO)O[C@@H](N2C=NC3=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N=CN=C32)[C@@H]1O | 3638.2 | Semi standard non polar | 33892256 | | 3'-AMP,3TBDMS,isomer #10 | CC(C)(C)[Si](C)(C)OP(=O)(O)O[C@@H]1[C@@H](CO)O[C@@H](N2C=NC3=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N=CN=C32)[C@@H]1O | 3696.3 | Standard non polar | 33892256 | | 3'-AMP,3TBDMS,isomer #10 | CC(C)(C)[Si](C)(C)OP(=O)(O)O[C@@H]1[C@@H](CO)O[C@@H](N2C=NC3=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N=CN=C32)[C@@H]1O | 4496.6 | Standard polar | 33892256 | | 3'-AMP,3TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO[Si](C)(C)C(C)(C)C)[C@@H](OP(=O)(O)O)[C@H]1O[Si](C)(C)C(C)(C)C | 3577.2 | Semi standard non polar | 33892256 | | 3'-AMP,3TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO[Si](C)(C)C(C)(C)C)[C@@H](OP(=O)(O)O)[C@H]1O[Si](C)(C)C(C)(C)C | 3657.7 | Standard non polar | 33892256 | | 3'-AMP,3TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO[Si](C)(C)C(C)(C)C)[C@@H](OP(=O)(O)O)[C@H]1O[Si](C)(C)C(C)(C)C | 4931.3 | Standard polar | 33892256 | | 3'-AMP,3TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@H](O)[C@@H]1OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 3616.0 | Semi standard non polar | 33892256 | | 3'-AMP,3TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@H](O)[C@@H]1OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 3520.3 | Standard non polar | 33892256 | | 3'-AMP,3TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@H](O)[C@@H]1OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 4777.6 | Standard polar | 33892256 | | 3'-AMP,3TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO[Si](C)(C)C(C)(C)C)[C@@H](OP(=O)(O)O[Si](C)(C)C(C)(C)C)[C@H]1O | 3620.7 | Semi standard non polar | 33892256 | | 3'-AMP,3TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO[Si](C)(C)C(C)(C)C)[C@@H](OP(=O)(O)O[Si](C)(C)C(C)(C)C)[C@H]1O | 3638.7 | Standard non polar | 33892256 | | 3'-AMP,3TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO[Si](C)(C)C(C)(C)C)[C@@H](OP(=O)(O)O[Si](C)(C)C(C)(C)C)[C@H]1O | 4762.3 | Standard polar | 33892256 | | 3'-AMP,3TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O)[C@@H]1OP(=O)(O)O | 3605.0 | Semi standard non polar | 33892256 | | 3'-AMP,3TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O)[C@@H]1OP(=O)(O)O | 3742.6 | Standard non polar | 33892256 | | 3'-AMP,3TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O)[C@@H]1OP(=O)(O)O | 4697.6 | Standard polar | 33892256 | | 3'-AMP,3TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@H](OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)[C@@H](CO)O[C@H]1N1C=NC2=C(N)N=CN=C21 | 3584.6 | Semi standard non polar | 33892256 | | 3'-AMP,3TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@H](OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)[C@@H](CO)O[C@H]1N1C=NC2=C(N)N=CN=C21 | 3508.7 | Standard non polar | 33892256 | | 3'-AMP,3TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@H](OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)[C@@H](CO)O[C@H]1N1C=NC2=C(N)N=CN=C21 | 4767.7 | Standard polar | 33892256 | | 3'-AMP,3TBDMS,isomer #7 | CC(C)(C)[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO)[C@@H](OP(=O)(O)O[Si](C)(C)C(C)(C)C)[C@H]1O[Si](C)(C)C(C)(C)C | 3602.9 | Semi standard non polar | 33892256 | | 3'-AMP,3TBDMS,isomer #7 | CC(C)(C)[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO)[C@@H](OP(=O)(O)O[Si](C)(C)C(C)(C)C)[C@H]1O[Si](C)(C)C(C)(C)C | 3634.2 | Standard non polar | 33892256 | | 3'-AMP,3TBDMS,isomer #7 | CC(C)(C)[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO)[C@@H](OP(=O)(O)O[Si](C)(C)C(C)(C)C)[C@H]1O[Si](C)(C)C(C)(C)C | 4734.1 | Standard polar | 33892256 | | 3'-AMP,3TBDMS,isomer #8 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@H](OP(=O)(O)O)[C@@H](CO)O[C@H]1N1C=NC2=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N=CN=C21 | 3612.3 | Semi standard non polar | 33892256 | | 3'-AMP,3TBDMS,isomer #8 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@H](OP(=O)(O)O)[C@@H](CO)O[C@H]1N1C=NC2=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N=CN=C21 | 3734.4 | Standard non polar | 33892256 | | 3'-AMP,3TBDMS,isomer #8 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@H](OP(=O)(O)O)[C@@H](CO)O[C@H]1N1C=NC2=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N=CN=C21 | 4671.1 | Standard polar | 33892256 | | 3'-AMP,3TBDMS,isomer #9 | CC(C)(C)[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO)[C@@H](OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)[C@H]1O | 3633.1 | Semi standard non polar | 33892256 | | 3'-AMP,3TBDMS,isomer #9 | CC(C)(C)[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO)[C@@H](OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)[C@H]1O | 3616.1 | Standard non polar | 33892256 | | 3'-AMP,3TBDMS,isomer #9 | CC(C)(C)[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO)[C@@H](OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)[C@H]1O | 4577.8 | Standard polar | 33892256 | | 3'-AMP,4TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 3738.4 | Semi standard non polar | 33892256 | | 3'-AMP,4TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 3590.5 | Standard non polar | 33892256 | | 3'-AMP,4TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 4670.5 | Standard polar | 33892256 | | 3'-AMP,4TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO[Si](C)(C)C(C)(C)C)[C@@H](OP(=O)(O)O[Si](C)(C)C(C)(C)C)[C@H]1O[Si](C)(C)C(C)(C)C | 3745.3 | Semi standard non polar | 33892256 | | 3'-AMP,4TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO[Si](C)(C)C(C)(C)C)[C@@H](OP(=O)(O)O[Si](C)(C)C(C)(C)C)[C@H]1O[Si](C)(C)C(C)(C)C | 3754.5 | Standard non polar | 33892256 | | 3'-AMP,4TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO[Si](C)(C)C(C)(C)C)[C@@H](OP(=O)(O)O[Si](C)(C)C(C)(C)C)[C@H]1O[Si](C)(C)C(C)(C)C | 4564.8 | Standard polar | 33892256 | | 3'-AMP,4TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1OP(=O)(O)O | 3720.6 | Semi standard non polar | 33892256 | | 3'-AMP,4TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1OP(=O)(O)O | 3848.4 | Standard non polar | 33892256 | | 3'-AMP,4TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1OP(=O)(O)O | 4415.7 | Standard polar | 33892256 | | 3'-AMP,4TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO[Si](C)(C)C(C)(C)C)[C@@H](OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)[C@H]1O | 3771.7 | Semi standard non polar | 33892256 | | 3'-AMP,4TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO[Si](C)(C)C(C)(C)C)[C@@H](OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)[C@H]1O | 3715.2 | Standard non polar | 33892256 | | 3'-AMP,4TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO[Si](C)(C)C(C)(C)C)[C@@H](OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)[C@H]1O | 4445.7 | Standard polar | 33892256 | | 3'-AMP,4TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O)[C@@H]1OP(=O)(O)O[Si](C)(C)C(C)(C)C | 3755.4 | Semi standard non polar | 33892256 | | 3'-AMP,4TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O)[C@@H]1OP(=O)(O)O[Si](C)(C)C(C)(C)C | 3812.2 | Standard non polar | 33892256 | | 3'-AMP,4TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)OC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O)[C@@H]1OP(=O)(O)O[Si](C)(C)C(C)(C)C | 4290.2 | Standard polar | 33892256 | | 3'-AMP,4TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO)[C@@H](OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)[C@H]1O[Si](C)(C)C(C)(C)C | 3756.0 | Semi standard non polar | 33892256 | | 3'-AMP,4TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO)[C@@H](OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)[C@H]1O[Si](C)(C)C(C)(C)C | 3731.5 | Standard non polar | 33892256 | | 3'-AMP,4TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)NC1=NC=NC2=C1N=CN2[C@@H]1O[C@H](CO)[C@@H](OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)[C@H]1O[Si](C)(C)C(C)(C)C | 4421.3 | Standard polar | 33892256 | | 3'-AMP,4TBDMS,isomer #7 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@H](OP(=O)(O)O[Si](C)(C)C(C)(C)C)[C@@H](CO)O[C@H]1N1C=NC2=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N=CN=C21 | 3749.9 | Semi standard non polar | 33892256 | | 3'-AMP,4TBDMS,isomer #7 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@H](OP(=O)(O)O[Si](C)(C)C(C)(C)C)[C@@H](CO)O[C@H]1N1C=NC2=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N=CN=C21 | 3823.5 | Standard non polar | 33892256 | | 3'-AMP,4TBDMS,isomer #7 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@H](OP(=O)(O)O[Si](C)(C)C(C)(C)C)[C@@H](CO)O[C@H]1N1C=NC2=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N=CN=C21 | 4268.4 | Standard polar | 33892256 | | 3'-AMP,4TBDMS,isomer #8 | CC(C)(C)[Si](C)(C)OP(=O)(O[C@@H]1[C@@H](CO)O[C@@H](N2C=NC3=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N=CN=C32)[C@@H]1O)O[Si](C)(C)C(C)(C)C | 3770.5 | Semi standard non polar | 33892256 | | 3'-AMP,4TBDMS,isomer #8 | CC(C)(C)[Si](C)(C)OP(=O)(O[C@@H]1[C@@H](CO)O[C@@H](N2C=NC3=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N=CN=C32)[C@@H]1O)O[Si](C)(C)C(C)(C)C | 3786.4 | Standard non polar | 33892256 | | 3'-AMP,4TBDMS,isomer #8 | CC(C)(C)[Si](C)(C)OP(=O)(O[C@@H]1[C@@H](CO)O[C@@H](N2C=NC3=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N=CN=C32)[C@@H]1O)O[Si](C)(C)C(C)(C)C | 4164.2 | Standard polar | 33892256 |
|
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental GC-MS | GC-MS Spectrum - 3'-AMP GC-EI-TOF (Non-derivatized) | splash10-0kuv-0973000000-eb7c71515eea7eb1a837 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - 3'-AMP GC-EI-TOF (Non-derivatized) | splash10-0kuv-0973000000-eb7c71515eea7eb1a837 | 2018-05-18 | HMDB team, MONA, MassBank | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 3'-AMP GC-MS (Non-derivatized) - 70eV, Positive | splash10-0002-9511000000-80f5030da41a88c5adf7 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 3'-AMP GC-MS (2 TMS) - 70eV, Positive | splash10-03xr-7973700000-7630afa3708fa5e5ab9e | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 3'-AMP GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental LC-MS/MS | LC-MS/MS Spectrum - 3'-AMP LC-ESI-ITFT , negative-QTOF | splash10-03di-2093000000-f9389aa723f687dcb780 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 3'-AMP LC-ESI-QTOF , negative-QTOF | splash10-01u1-9463000000-deedf4a3a159267bf008 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 3'-AMP LC-ESI-ITFT , positive-QTOF | splash10-000i-1900000000-3070b457af9f8caf95ae | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 3'-AMP LC-ESI-QTOF , positive-QTOF | splash10-000i-0901000000-01666f7ac8779350ae53 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 3'-AMP 50V, Negative-QTOF | splash10-03di-2093000000-e566bf05f29e5c2f202b | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 3'-AMP 50V, Positive-QTOF | splash10-000i-1900000000-796d8e13194f52dee246 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 3'-AMP 35V, Positive-QTOF | splash10-000i-0900000000-02e4343bf1e5fbc63510 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 3'-AMP 35V, Negative-QTOF | splash10-0002-0009000000-38164510e452a6ab6012 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3'-AMP 10V, Positive-QTOF | splash10-000i-2913000000-583726ba8cf3132ced34 | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3'-AMP 20V, Positive-QTOF | splash10-000i-0900000000-3743b8381504a528e937 | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3'-AMP 40V, Positive-QTOF | splash10-000i-3900000000-674aca6f0bee774491a7 | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3'-AMP 10V, Negative-QTOF | splash10-0032-4709000000-0e304c018489ee824b4e | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3'-AMP 20V, Negative-QTOF | splash10-001i-3900000000-28c48abec776120de260 | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3'-AMP 40V, Negative-QTOF | splash10-004i-9500000000-64e6e28bb636afabd554 | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3'-AMP 10V, Negative-QTOF | splash10-0002-0009000000-f5a4678a70e90bab759b | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3'-AMP 20V, Negative-QTOF | splash10-001i-1921000000-253481bcb5e35615598c | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3'-AMP 40V, Negative-QTOF | splash10-003r-8900000000-3b498c0da7159391cb24 | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3'-AMP 10V, Positive-QTOF | splash10-0002-0009000000-97735d217b8d45d68457 | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3'-AMP 20V, Positive-QTOF | splash10-000i-0921000000-e877995fe049396ac137 | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 3'-AMP 40V, Positive-QTOF | splash10-000i-0900000000-fc6f39d328b9fab00fc1 | 2021-09-24 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum |
|
|---|