| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected and Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2021-09-14 14:57:20 UTC |
|---|
| HMDB ID | HMDB0000217 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
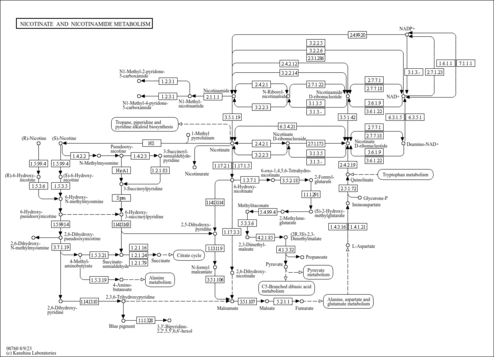
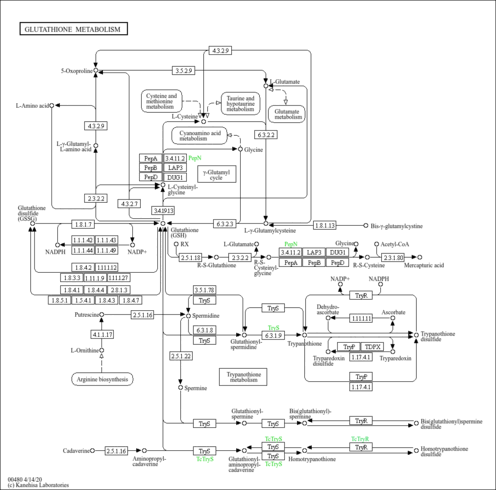
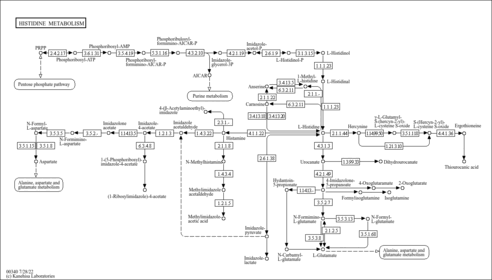
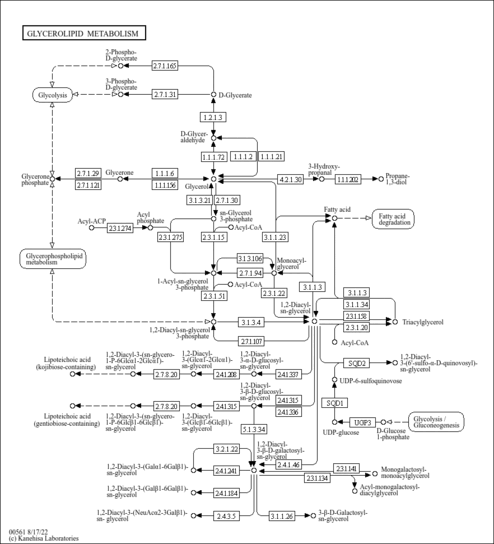
| Common Name | NADP |
|---|
| Description | Nicotinamide adenine dinucleotide phosphate (NADP) is a coenzyme composed of ribosylnicotinamide 5'-phosphate (NMN) coupled with a pyrophosphate linkage to 5'-phosphate adenosine 2',5'-bisphosphate. NADP serves as an electron carrier in a number of reactions, being alternately oxidized (NADP+) and reduced (NADPH). NADP is formed through the addition of a phosphate group to the 2' position of the adenosyl nucleotide through an ester linkage (Dorland, 27th ed). This extra phosphate is added by the enzyme NAD+ kinase and removed via NADP+ phosphatase. NADP is also known as TPN (triphosphopyridine nucleotide) and it is an important cofactor used in anabolic reactions in all forms of cellular life. Examples include the Calvin cycle, cholesterol synthesis, fatty acid elongation, and nucleic acid synthesis (Wikipedia ). |
|---|
| Structure | NC(=O)C1=C[N+](=CC=C1)[C@@H]1O[C@H](COP([O-])(=O)OP(O)(=O)OC[C@H]2O[C@H]([C@H](OP(O)(O)=O)[C@@H]2O)N2C=NC3=C2N=CN=C3N)[C@@H](O)[C@H]1O InChI=1S/C21H28N7O17P3/c22-17-12-19(25-7-24-17)28(8-26-12)21-16(44-46(33,34)35)14(30)11(43-21)6-41-48(38,39)45-47(36,37)40-5-10-13(29)15(31)20(42-10)27-3-1-2-9(4-27)18(23)32/h1-4,7-8,10-11,13-16,20-21,29-31H,5-6H2,(H7-,22,23,24,25,32,33,34,35,36,37,38,39)/t10-,11-,13-,14-,15-,16-,20-,21-/m1/s1 |
|---|
| Synonyms | | Value | Source |
|---|
| Codehydrase II | ChEBI | | Codehydrogenase II | ChEBI | | Coenzyme II | ChEBI | | NAD phosphate | ChEBI | | NADP nicotinamide-adenine-dinucleotide phosphATE | ChEBI | | TPN | ChEBI | | Triphosphopyridine nucleotide | ChEBI | | NAD phosphoric acid | Generator | | NADP nicotinamide-adenine-dinucleotide phosphoric acid | Generator | | Dinucleotide phosphate, nicotinamide-adenine | HMDB | | NADPH | HMDB | | Nicotinamide-adenine dinucleotide phosphate | HMDB | | Nicotinamide adenine dinucleotide phosphate | HMDB | | Nucleotide, triphosphopyridine | HMDB | | Phosphate, nicotinamide-adenine dinucleotide | HMDB | | Adenine-nicotinamide dinucleotide phosphate | HMDB | | NADP+ | HMDB | | beta-NADP | HMDB | | beta-Nicotinamide adenine dinucleotide phosphate | HMDB | | beta-TPN | HMDB | | Β-nadp | HMDB | | Β-nicotinamide adenine dinucleotide phosphate | HMDB | | Β-TPN | HMDB | | NADP | ChEBI, MeSH |
|
|---|
| Chemical Formula | C21H28N7O17P3 |
|---|
| Average Molecular Weight | 743.405 |
|---|
| Monoisotopic Molecular Weight | 743.075452041 |
|---|
| IUPAC Name | 1-[(2R,3R,4S,5R)-5-{[({[(2R,3R,4R,5R)-5-(6-amino-9H-purin-9-yl)-3-hydroxy-4-(phosphonooxy)oxolan-2-yl]methoxy}(hydroxy)phosphoryl phosphono)oxy]methyl}-3,4-dihydroxyoxolan-2-yl]-3-carbamoyl-1lambda5-pyridin-1-ylium |
|---|
| Traditional Name | 1-[(2R,3R,4S,5R)-5-[({[(2R,3R,4R,5R)-5-(6-aminopurin-9-yl)-3-hydroxy-4-(phosphonooxy)oxolan-2-yl]methoxy(hydroxy)phosphoryl phosphono}oxy)methyl]-3,4-dihydroxyoxolan-2-yl]-3-carbamoyl-1lambda5-pyridin-1-ylium |
|---|
| CAS Registry Number | 53-59-8 |
|---|
| SMILES | NC(=O)C1=C[N+](=CC=C1)[C@@H]1O[C@H](COP([O-])(=O)OP(O)(=O)OC[C@H]2O[C@H]([C@H](OP(O)(O)=O)[C@@H]2O)N2C=NC3=C2N=CN=C3N)[C@@H](O)[C@H]1O |
|---|
| InChI Identifier | InChI=1S/C21H28N7O17P3/c22-17-12-19(25-7-24-17)28(8-26-12)21-16(44-46(33,34)35)14(30)11(43-21)6-41-48(38,39)45-47(36,37)40-5-10-13(29)15(31)20(42-10)27-3-1-2-9(4-27)18(23)32/h1-4,7-8,10-11,13-16,20-21,29-31H,5-6H2,(H7-,22,23,24,25,32,33,34,35,36,37,38,39)/t10-,11-,13-,14-,15-,16-,20-,21-/m1/s1 |
|---|
| InChI Key | XJLXINKUBYWONI-NNYOXOHSSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as catechols. Catechols are compounds containing a 1,2-benzenediol moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Phenols |
|---|
| Sub Class | Benzenediols |
|---|
| Direct Parent | Catechols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Catechol
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Aralkylamine
- Monocyclic benzene moiety
- 1,2-aminoalcohol
- Secondary alcohol
- Organic nitrogen compound
- Aromatic alcohol
- Hydrocarbon derivative
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Primary aliphatic amine
- Organopnictogen compound
- Organic oxygen compound
- Amine
- Alcohol
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Not Available | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Not Available |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Kovats Retention IndicesUnderivatized |
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - NADP GC-MS (TMS_1_1) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADP GC-MS (TMS_1_2) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADP GC-MS (TMS_1_3) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADP GC-MS (TMS_1_4) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADP GC-MS (TMS_1_5) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADP GC-MS (TMS_1_6) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADP GC-MS (TMS_1_7) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADP GC-MS (TMS_2_1) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADP GC-MS (TMS_2_2) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADP GC-MS (TMS_2_3) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADP GC-MS (TMS_2_4) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADP GC-MS (TMS_2_5) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADP GC-MS (TMS_2_6) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADP GC-MS (TMS_2_7) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADP GC-MS (TMS_2_8) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADP GC-MS (TMS_2_9) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADP GC-MS (TMS_2_10) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADP GC-MS (TMS_2_11) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADP GC-MS (TMS_2_12) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADP GC-MS (TMS_2_13) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADP GC-MS (TMS_2_14) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADP GC-MS (TMS_2_15) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADP GC-MS (TMS_2_16) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADP GC-MS (TMS_2_17) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - NADP GC-MS (TMS_2_18) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental LC-MS/MS | LC-MS/MS Spectrum - NADP LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive-QTOF | splash10-00dl-0219003700-2f52e3c5db41066a112c | 2019-11-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NADP LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive-QTOF | splash10-0uk9-0301009000-a3d0c464e56f6e320c80 | 2019-11-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NADP LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive-QTOF | splash10-00dl-0000090000-c18a7719161c63a71938 | 2019-11-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NADP LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive-QTOF | splash10-0udi-0000009000-1dde5b221786fe375304 | 2019-11-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NADP LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive-QTOF | splash10-00dl-0209000700-02057593d6470f6a2075 | 2019-11-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NADP LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive-QTOF | splash10-0uk9-0301109000-bf4996084c09a9176489 | 2019-11-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NADP LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive-QTOF | splash10-0uk9-0301009000-b3e8d304dfdc4711e0ce | 2019-11-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NADP LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive-QTOF | splash10-0udi-0000009000-384d275a638928dd40f1 | 2019-11-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NADP LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative-QTOF | splash10-00dl-0000229600-bd164bae0cc54f45b961 | 2019-11-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NADP LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative-QTOF | splash10-004i-0027900000-25e0180b7638ed18e207 | 2019-11-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NADP LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative-QTOF | splash10-0a4i-0011953000-c328377f842fac60b55e | 2019-11-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NADP LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative-QTOF | splash10-00di-0000009000-0ec3670e706b086d382a | 2019-11-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NADP LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative-QTOF | splash10-00dl-0000109700-df2a731fd5f55e9e7a8e | 2019-11-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NADP LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative-QTOF | splash10-0a4i-0011953000-981d13d6b50f18ebc910 | 2019-11-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NADP LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative-QTOF | splash10-00di-0000009000-f9526262833104be0a2e | 2019-11-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - NADP LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative-QTOF | splash10-00di-0000009000-c0cedd489befb2073370 | 2019-11-22 | HMDB team, MONA | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - NADP 10V, Positive-QTOF | splash10-0006-0000000900-d510a9264bee14bd85ee | 2017-07-25 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - NADP 20V, Positive-QTOF | splash10-000t-0000000900-fb29571fb864b5c4179b | 2017-07-25 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - NADP 40V, Positive-QTOF | splash10-006y-2910001000-5066541857e9b5d8bbbf | 2017-07-25 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - NADP 10V, Negative-QTOF | splash10-0006-1000000900-8cdcef7ab2fa3bdd51e1 | 2017-07-26 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - NADP 20V, Negative-QTOF | splash10-0007-6100000900-396416b5dd4d0892e43d | 2017-07-26 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - NADP 40V, Negative-QTOF | splash10-005a-9100000000-e5765145791ae2971a89 | 2017-07-26 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - NADP 10V, Positive-QTOF | splash10-000i-0900001200-ff4ca74a6b585e359275 | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - NADP 20V, Positive-QTOF | splash10-000i-0901113400-26884ea59b81b6581275 | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - NADP 40V, Positive-QTOF | splash10-000j-2902210000-acc59733c27dab4bbf95 | 2021-09-23 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Experimental 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | 2019-11-22 | Wishart Lab | View Spectrum |
|
|---|