| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected and Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2022-03-07 02:49:00 UTC |
|---|
| HMDB ID | HMDB0000222 |
|---|
| Secondary Accession Numbers | - HMDB0000846
- HMDB00222
- HMDB00846
|
|---|
| Metabolite Identification |
|---|
| Common Name | Palmitoylcarnitine |
|---|
| Description | L-Palmitoylcarnitine or hexadecanoylcarnitine is an acylcarnitine. It is technically a long-chain acyl fatty acid derivative ester of carnitine which facilitates the transfer of long-chain fatty acids from cytoplasm into mitochondria during the oxidation of fatty acids. The general role of acylcarnitines is to transport acyl-groups, organic acids and fatty acids, from the cytoplasm into the mitochondria so that they can be broken down to produce energy. As part of this process, palmitic acid is first transported into the cell via the long-chain fatty acid transport protein 1 (FATP1). Once inside the cell it undergoes a reaction to form an acyl-CoA derivative called palmitoyl-CoA. This reaction is facilitated by the Long-chain fatty-acid CoA ligase 1 protein, which adds a CoA moiety to appropriate acyl groups. Many acyl-CoA groups will then further react with other zwitterionic compounds such as carnitine (to form acylcarnitines) and amino acids (to form acyl amides). The carnitine needed to form acylcarnitines inside the cell is transported into the cell by the organic cation/carnitine transporter 2. In forming an acylcarnitine derivative, palmitoyl-CoA reacts with L-carnitine to form palmitoylcarnitine. This reaction is catalyzed by carnitine O-palmitoyltransferase. This enzyme resides in the mitochondrial outer membrane. While this reaction takes place, the palmitoylcarnitine is moved into the mitochondrial intermembrane space. Following the reaction, the newly synthesized acylcarnitine is transported into the mitochondrial matrix by a mitochondrial carnitine/acylcarnitine carrier protein found in the mitochondrial inner membrane. Once in the matrix, palmitoylcarnitine can react with the carnitine O-palmitoyltransferase 2 enzyme found in the mitochondrial inner membrane to once again form palmitoyl-CoA and L-carnitine. Palmitoyl-CoA then enters into the mitochondrial beta-oxidation pathway to form aceytl-CoA. Acetyl-CoA can go on to enter the TCA cycle, or it can react with L-carnitine to form L-acetylcarnitine in a reaction catalyzed by Carnitine O-acetyltransferase. This reaction can occur in both directions, and L-acetylcarnitine and CoA can react to form acetyl-CoA and L-carnitine in certain circumstances. Finally, acetyl-CoA in the cytosol can be catalyzed by acetyl-CoA carboxylase 1 to form malonyl-CoA, which inhibits the action of carnitine O-palmitoyltransferase 1, thereby preventing palmitoylcarnitine from forming and thereby preventing it from being transported into the mitochondria. L-Palmitoylcarnitine has been also reported to change the activity of certain proteins and to stimulate the activity of caspases 3, 7, and 8. Interestingly, the level of this long-chain acylcarnitine has been shown to increase during apoptosis. Palmitoylcarnitine has also been reported to diminish the binding of phorbol esters (protein kinase C activators) and the autophosphorylation of the enzyme. Some of the physicochemical properties of palmitoylcarnitine may help to explain the need for coenzyme A-carnitine-coenzyme A acyl exchange during mitochondrial fatty acid import. The amphiphilic character of palmitoylcarnitine may also explain its proposed involvement in the pathogenesis of myocardial ischemia. L-Palmitoylcarnitine accumulates in ischemic myocardium and potentially contributes to myocardial damage through alterations in membrane molecular dynamics. This is a mechanism through which could play an important role in ischemic injury (PMID: 2540838 , 15363641 , 8706815 ). Palmitoylcarnitine is characteristically elevated in late-onset carnitine palmitoyltransferase II deficiency (OMIM: 255110 ). |
|---|
| Structure | CCCCCCCCCCCCCCCC(=O)O[C@H](CC([O-])=O)C[N+](C)(C)C InChI=1S/C23H45NO4/c1-5-6-7-8-9-10-11-12-13-14-15-16-17-18-23(27)28-21(19-22(25)26)20-24(2,3)4/h21H,5-20H2,1-4H3/t21-/m1/s1 |
|---|
| Synonyms | | Value | Source |
|---|
| (3R)-3-(Hexadecanoyloxy)-4-(trimethylazaniumyl)butanoate | ChEBI | | (3R)-3-Palmitoyloxy-4-(trimethylammonio)butanoate | ChEBI | | Hexadecanoyl-L-carnitine | ChEBI | | Hexadecanoylcarnitine | ChEBI | | Hexadecenoyl carnitine | ChEBI | | L-Carnitine palmitoyl ester | ChEBI | | O-Hexadecanoyl-(R)-carnitine | ChEBI | | O-Hexadecanoyl-R-carnitine | ChEBI | | Palmitoyl-L-carnitine | ChEBI | | Palmitoylcarnitine | ChEBI | | (3R)-3-(Hexadecanoyloxy)-4-(trimethylazaniumyl)butanoic acid | Generator | | (3R)-3-Palmitoyloxy-4-(trimethylammonio)butanoic acid | Generator | | (+)-Palmitoylcarnitine | HMDB | | 3-Carboxy-N,N,N-trimethyl-2-[(1-oxohexadecyl)oxy]-1-propanaminium | HMDB | | L(-)-Palmitylcarnitine | HMDB | | L-Palmitoyl-L-carnitine | HMDB | | Palmitoyl-(-)-carnitine | HMDB | | Palmityl-L-carnitine | HMDB | | Palmitylcarnitine | HMDB | | (2R)-Palmitoylcarnitine | HMDB | | C16 Carnitine | HMDB | | L-Palmitoylcarnitine | HMDB |
|
|---|
| Chemical Formula | C23H46NO4 |
|---|
| Average Molecular Weight | 400.623 |
|---|
| Monoisotopic Molecular Weight | 400.342135385 |
|---|
| IUPAC Name | (3R)-3-(hexadecanoyloxy)-4-(trimethylazaniumyl)butanoate |
|---|
| Traditional Name | palmitoylcarnitine |
|---|
| CAS Registry Number | 2364-67-2 |
|---|
| SMILES | CCCCCCCCCCCCCCCC(=O)O[C@H](CC(O)=O)C[N+](C)(C)C |
|---|
| InChI Identifier | InChI=1S/C23H45NO4/c1-5-6-7-8-9-10-11-12-13-14-15-16-17-18-23(27)28-21(19-22(25)26)20-24(2,3)4/h21H,5-20H2,1-4H3/p+1/t21-/m1/s1 |
|---|
| InChI Key | XOMRRQXKHMYMOC-OAQYLSRUSA-O |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as acyl carnitines. These are organic compounds containing a fatty acid with the carboxylic acid attached to carnitine through an ester bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acid esters |
|---|
| Direct Parent | Acyl carnitines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Acyl-carnitine
- Dicarboxylic acid or derivatives
- Tetraalkylammonium salt
- Quaternary ammonium salt
- Carboxylic acid ester
- Carboxylic acid salt
- Carboxylic acid derivative
- Carboxylic acid
- Organic nitrogen compound
- Organooxygen compound
- Organonitrogen compound
- Organic salt
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Carbonyl group
- Amine
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Not Available |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross Sections| Predictor | Adduct Type | CCS Value (Å2) | Reference |
|---|
| DeepCCS | [M+H]+ | 214.767 | 30932474 | | DeepCCS | [M-H]- | 210.748 | 30932474 | | DeepCCS | [M-2H]- | 247.291 | 30932474 | | DeepCCS | [M+Na]+ | 223.582 | 30932474 |
Predicted Kovats Retention IndicesUnderivatizedDerivatized |
|---|
| Spectra |
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - Palmitoylcarnitine GC-MS (Non-derivatized) - 70eV, Positive | splash10-00di-9220000000-890219021b84f6894540 | 2016-09-22 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Palmitoylcarnitine GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Palmitoylcarnitine GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Palmitoylcarnitine GC-MS (TMS_1_1) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Palmitoylcarnitine GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental LC-MS/MS | LC-MS/MS Spectrum - Palmitoylcarnitine Quattro_QQQ 10V, Positive-QTOF (Annotated) | splash10-0udi-1000900000-109f35c9819eb5eed7b7 | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Palmitoylcarnitine Quattro_QQQ 25V, Positive-QTOF (Annotated) | splash10-000i-9000000000-9d6bb14eddb4cfa8341a | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Palmitoylcarnitine Quattro_QQQ 40V, Positive-QTOF (Annotated) | splash10-000i-9000000000-87b1d0debe4e0cb7bd31 | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Palmitoylcarnitine Linear Ion Trap , negative-QTOF | splash10-014i-0139500000-21c36c851939b94d4523 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Palmitoylcarnitine Linear Ion Trap , negative-QTOF | splash10-014i-0029600000-69ebe292c2ce42c8d810 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Palmitoylcarnitine Linear Ion Trap , positive-QTOF | splash10-0f6x-0139600000-197944fd1aa4ac44bdd2 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Palmitoylcarnitine Linear Ion Trap , positive-QTOF | splash10-0uxr-0000613900-006582b143e5906e51cc | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Palmitoylcarnitine Linear Ion Trap , positive-QTOF | splash10-0uxr-0000614900-14a5d773f652b71671fb | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Palmitoylcarnitine Linear Ion Trap , positive-QTOF | splash10-0f6x-0139600000-b0528be9c13e412551af | 2017-09-14 | HMDB team, MONA | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Palmitoylcarnitine 10V, Positive-QTOF | splash10-0udi-0000900000-b54b0a7f2164f15f15f2 | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Palmitoylcarnitine 20V, Positive-QTOF | splash10-0f79-9000500000-d7434e08a3e0942fa2e9 | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Palmitoylcarnitine 40V, Positive-QTOF | splash10-000i-9000000000-e9262cbaff8cb4ad0ba6 | 2021-09-24 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Experimental 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, CD3OD, experimental) | 2012-12-05 | Wishart Lab | View Spectrum |
|
|---|
| Biological Properties |
|---|
| Cellular Locations | - Cytoplasm
- Extracellular
- Membrane
- Mitochondria
|
|---|
| Biospecimen Locations | |
|---|
| Tissue Locations | |
|---|
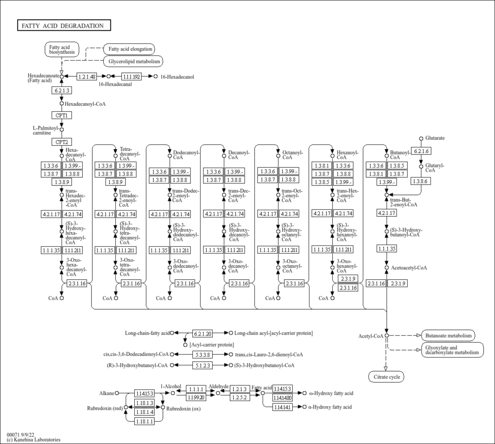
| Pathways | |
|---|
| Normal Concentrations |
|---|
| |
| Blood | Detected and Quantified | 0.153 (0.073-0.227) uM | Children (1-13 years old) | Both | Normal | | details | | Blood | Detected and Quantified | 0.113 +/- 0.006 uM | Adult (>18 years old) | Both | Normal | | details | | Blood | Detected and Quantified | 0.050-0.180 uM | Adult (>18 years old) | Both | Normal | | details | | Blood | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Blood | Detected and Quantified | 0.15 +/- 0.17 uM | Children (1-13 years old) | Both | Normal | | details | | Blood | Detected and Quantified | 0.06 +/- 0.04 uM | Children (1-13 years old) | Both | Normal | | details | | Blood | Detected and Quantified | 0.11 +/- 0.03 uM | Adult (>18 years old) | Both | Normal | | details | | Blood | Detected and Quantified | 0.12(0.03) uM | Adult (>18 years old) | Both | Normal | | details | | Blood | Detected and Quantified | 0.050-0.180 uM | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected and Quantified | 0.36 +/- 0.27 nmol/g wet feces | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected and Quantified | 0.22 +/- 0.07 nmol/g wet feces | Adult (>18 years old) | Both | Normal | | details | | Saliva | Detected and Quantified | 0.034 +/- 0.012 uM | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected and Quantified | 0.01-0.06 umol/mmol creatinine | Newborn (0-30 days old) | Both | Normal | | details | | Urine | Detected and Quantified | 0.01(0.01-0.07) umol/mmol creatinine | Newborn (0-30 days old) | Female | Normal | | details | | Urine | Detected and Quantified | 0.01(0.01-0.03) umol/mmol creatinine | Newborn (0-30 days old) | Male | Normal | | details | | Urine | Detected and Quantified | 0.0022 (0.0020-0.0030) umol/mmol creatinine | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected and Quantified | 0.006 +/- 0.0018 umol/mmol creatinine | Children (1 - 13 years old) | Not Specified | Normal | | details | | Urine | Detected and Quantified | 0.5 umol/mmol creatinine | Adult (>18 years old) | Both | Normal | | details |
|
|---|
| Abnormal Concentrations |
|---|
| |
| Blood | Detected and Quantified | 1.909 (1.012-2.233) uM | Adult (>18 years old) | Both | Very long-chain acyl-CoA dehydrogenase deficiency (vLCAD) | | details | | Blood | Detected and Quantified | 0.097 +/- 0.006 uM | Adult (>18 years old) | Both | Celiac disease | | details | | Blood | Detected and Quantified | 0.13 +/- 0.09 uM | Children (1-13 years old) | Both | Acetaminophen overdose | | details | | Blood | Detected and Quantified | 0.046 (0.0375) uM | Adult (>18 years old) | Female | Pregnancy with fetus having congenital heart defect | | details | | Blood | Detected and Quantified | 0.14(0.04) uM | Adult (>18 years old) | Both | Heart failure with preserved ejection fraction | | details | | Blood | Detected and Quantified | 0.096 +/- 0.032 uM | Children (1-13 years old) | Both | Obesity | | details | | Blood | Detected and Quantified | 0.101 +/- 0.026 uM | Children (1-13 years old) | Both | Obesity | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Colorectal cancer | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | liver cirrhosis | | details | | Urine | Detected and Quantified | 0.0046 +/- 0.0018 umol/mmol creatinine | Children (1 - 13 years old) | Not Specified | Eosinophilic esophagitis | | details | | Urine | Detected and Quantified | 0.0064 +/- 0.0047 umol/mmol creatinine | Children (1 - 13 years old) | Not Specified | Gastroesophageal reflux disease | | details |
|
|---|
| Associated Disorders and Diseases |
|---|
| Disease References | | Celiac disease |
|---|
- Bene J, Komlosi K, Gasztonyi B, Juhasz M, Tulassay Z, Melegh B: Plasma carnitine ester profile in adult celiac disease patients maintained on long-term gluten free diet. World J Gastroenterol. 2005 Nov 14;11(42):6671-5. [PubMed:16425363 ]
| | Very Long Chain Acyl-CoA Dehydrogenase Deficiency |
|---|
- Costa CG, Struys EA, Bootsma A, ten Brink HJ, Dorland L, Tavares de Almeida I, Duran M, Jakobs C: Quantitative analysis of plasma acylcarnitines using gas chromatography chemical ionization mass fragmentography. J Lipid Res. 1997 Jan;38(1):173-82. [PubMed:9034211 ]
| | Obesity |
|---|
- Simone Wahl, Christina Holzapfel, Zhonghao Yu, Michaela Breier, Ivan Kondofersky, Christiane Fuchs, Paula Singmann, Cornelia Prehn, Jerzy Adamski, Harald Grallert, Thomas Illig, Rui Wang-Sattler, Thomas Reinehr (2013). Metabolomics reveals determinants of weight loss during lifestyle intervention in obese children. Metabolomics.
| | Cirrhosis |
|---|
- Huang HJ, Zhang AY, Cao HC, Lu HF, Wang BH, Xie Q, Xu W, Li LJ: Metabolomic analyses of faeces reveals malabsorption in cirrhotic patients. Dig Liver Dis. 2013 Aug;45(8):677-82. doi: 10.1016/j.dld.2013.01.001. Epub 2013 Feb 4. [PubMed:23384618 ]
| | Colorectal cancer |
|---|
- Goedert JJ, Sampson JN, Moore SC, Xiao Q, Xiong X, Hayes RB, Ahn J, Shi J, Sinha R: Fecal metabolomics: assay performance and association with colorectal cancer. Carcinogenesis. 2014 Sep;35(9):2089-96. doi: 10.1093/carcin/bgu131. Epub 2014 Jul 18. [PubMed:25037050 ]
| | Eosinophilic esophagitis |
|---|
- Slae, M., Huynh, H., Wishart, D.S. (2014). Analysis of 30 normal pediatric urine samples via NMR spectroscopy (unpublished work). NA.
|
|
|---|
| Associated OMIM IDs | - 212750 (Celiac disease)
- 201475 (Very Long Chain Acyl-CoA Dehydrogenase Deficiency)
- 601665 (Obesity)
- 114500 (Colorectal cancer)
- 610247 (Eosinophilic esophagitis)
|
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| Phenol Explorer Compound ID | Not Available |
|---|
| FooDB ID | FDB021910 |
|---|
| KNApSAcK ID | Not Available |
|---|
| Chemspider ID | 10128117 |
|---|
| KEGG Compound ID | C02990 |
|---|
| BioCyc ID | CPD-419 |
|---|
| BiGG ID | 40966 |
|---|
| Wikipedia Link | Palmitoylcarnitine |
|---|
| METLIN ID | 5231 |
|---|
| PubChem Compound | 11953816 |
|---|
| PDB ID | Not Available |
|---|
| ChEBI ID | 17490 |
|---|
| Food Biomarker Ontology | Not Available |
|---|
| VMH ID | PMTCRN |
|---|
| MarkerDB ID | Not Available |
|---|
| Good Scents ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Norum, Kaare R. Palmityl coenzyme A-carnitine palmityltransferase. Purification from calf-liver mitochondria and some properties of the enzyme. Biochimica et Biophysica Acta, Specialized Section on Enzymological Subjects (1964), 89(1), 95-108. |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| General References | - Kamimori H, Hamashima Y, Konishi M: Determination of carnitine and saturated-acyl group carnitines in human urine by high-performance liquid chromatography with fluorescence detection. Anal Biochem. 1994 May 1;218(2):417-24. [PubMed:8074302 ]
- Mueller P, Schulze A, Schindler I, Ethofer T, Buehrdel P, Ceglarek U: Validation of an ESI-MS/MS screening method for acylcarnitine profiling in urine specimens of neonates, children, adolescents and adults. Clin Chim Acta. 2003 Jan;327(1-2):47-57. [PubMed:12482618 ]
- Moder M, Kiessling A, Loster H, Bruggemann L: The pattern of urinary acylcarnitines determined by electrospray mass spectrometry: a new tool in the diagnosis of diabetes mellitus. Anal Bioanal Chem. 2003 Jan;375(2):200-10. Epub 2003 Jan 4. [PubMed:12560963 ]
- Wasant P, Matsumoto I, Naylor E, Liammongkolkul S: Mitochondrial fatty acid oxidation disorders in Thai infants: a report of 3 cases. J Med Assoc Thai. 2002 Aug;85 Suppl 2:S710-9. [PubMed:12403251 ]
- Young SP, Matern D, Gregersen N, Stevens RD, Bali D, Liu HM, Koeberl DD, Millington DS: A comparison of in vitro acylcarnitine profiling methods for the diagnosis of classical and variant short chain acyl-CoA dehydrogenase deficiency. Clin Chim Acta. 2003 Nov;337(1-2):103-13. [PubMed:14568186 ]
- Poorthuis BJ, Jille-Vlckova T, Onkenhout W: Determination of acylcarnitines in urine of patients with inborn errors of metabolism using high-performance liquid chromatography after derivatization with 4'-bromophenacylbromide. Clin Chim Acta. 1993 Jul 16;216(1-2):53-61. [PubMed:8222273 ]
- Bhuiyan AK, Jackson S, Turnbull DM, Aynsley-Green A, Leonard JV, Bartlett K: The measurement of carnitine and acyl-carnitines: application to the investigation of patients with suspected inherited disorders of mitochondrial fatty acid oxidation. Clin Chim Acta. 1992 May 15;207(3):185-204. [PubMed:1327583 ]
- Nalecz KA, Miecz D, Berezowski V, Cecchelli R: Carnitine: transport and physiological functions in the brain. Mol Aspects Med. 2004 Oct-Dec;25(5-6):551-67. [PubMed:15363641 ]
- Watanabe H, Kobayashi A, Hayashi H, Yamazaki N: Effects of long-chain acyl carnitine on membrane fluidity of human erythrocytes. Biochim Biophys Acta. 1989 Apr 28;980(3):315-8. [PubMed:2540838 ]
- Goni FM, Requero MA, Alonso A: Palmitoylcarnitine, a surface-active metabolite. FEBS Lett. 1996 Jul 15;390(1):1-5. [PubMed:8706815 ]
- Elshenawy S, Pinney SE, Stuart T, Doulias PT, Zura G, Parry S, Elovitz MA, Bennett MJ, Bansal A, Strauss JF 3rd, Ischiropoulos H, Simmons RA: The Metabolomic Signature of the Placenta in Spontaneous Preterm Birth. Int J Mol Sci. 2020 Feb 4;21(3). pii: ijms21031043. doi: 10.3390/ijms21031043. [PubMed:32033212 ]
|
|---|