| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected and Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2022-03-07 02:49:00 UTC |
|---|
| HMDB ID | HMDB0000268 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Tetrahydrocorticosterone |
|---|
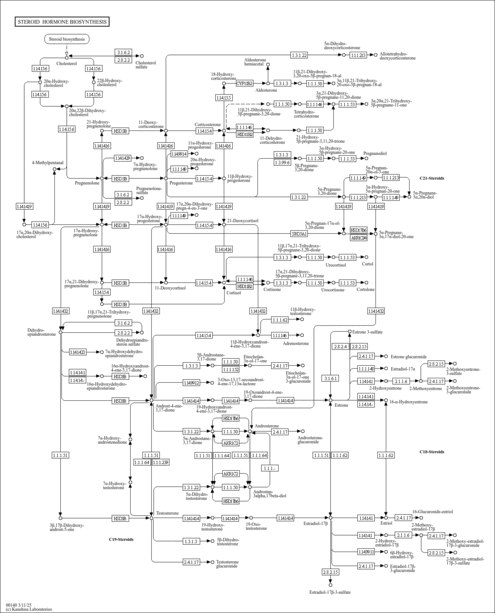
| Description | Tetrahydrocorticosterone belongs to the class of organic compounds known as 21-hydroxysteroids. These are steroids carrying a hydroxyl group at the 21-position of the steroid backbone. Thus, tetrahydrocorticosterone is considered to be a steroid lipid molecule. Tetrahydrocorticosterone is a very hydrophobic molecule, practically insoluble (in water), and relatively neutral. Tetrahydrocorticosterone is one of the major urinary metabolites from corticosterone. Premenopausal patients with early breast cancer excrete subnormal amounts of tetrahydrocorticosterone as compared with the normal subjects of corresponding ages (PMID: 1133844 ). |
|---|
| Structure | [H][C@@]12CC[C@H](C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]1([H])[C@@]2([H])CC[C@]2([H])C[C@H](O)CC[C@]12C InChI=1S/C21H34O4/c1-20-8-7-13(23)9-12(20)3-4-14-15-5-6-16(18(25)11-22)21(15,2)10-17(24)19(14)20/h12-17,19,22-24H,3-11H2,1-2H3/t12-,13-,14+,15+,16-,17+,19-,20+,21+/m1/s1 |
|---|
| Synonyms | | Value | Source |
|---|
| (3alpha,5beta,11beta)-3,11,21-Trihydroxypregnan-20-one | HMDB | | (3α,5β,11β)-3,11,21-Trihydroxypregnan-20-one | HMDB | | 3alpha,11beta,21-Trihydroxy-5beta-pregnan-20-one | HMDB | | 3alpha,5beta-Tetrahydrocorticosterone | HMDB | | 3α,11β,21-Trihydroxy-5β-pregnan-20-one | HMDB | | 3α,5β-Tetrahydrocorticosterone | HMDB | | 5beta-Pregnane-3alpha,11beta,21-triol-20-one | HMDB | | 5β-Pregnane-3α,11β,21-triol-20-one | HMDB | | THB | HMDB | | Tetrahydrocorticosterone | HMDB |
|
|---|
| Chemical Formula | C21H34O4 |
|---|
| Average Molecular Weight | 350.4923 |
|---|
| Monoisotopic Molecular Weight | 350.245709576 |
|---|
| IUPAC Name | 1-[(1S,2S,5R,7R,10S,11S,14S,15S,17S)-5,17-dihydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadecan-14-yl]-2-hydroxyethan-1-one |
|---|
| Traditional Name | 1-[(1S,2S,5R,7R,10S,11S,14S,15S,17S)-5,17-dihydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadecan-14-yl]-2-hydroxyethanone |
|---|
| CAS Registry Number | 68-42-8 |
|---|
| SMILES | [H][C@@]12CC[C@H](C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]1([H])[C@@]2([H])CC[C@]2([H])C[C@H](O)CC[C@]12C |
|---|
| InChI Identifier | InChI=1S/C21H34O4/c1-20-8-7-13(23)9-12(20)3-4-14-15-5-6-16(18(25)11-22)21(15,2)10-17(24)19(14)20/h12-17,19,22-24H,3-11H2,1-2H3/t12-,13-,14+,15+,16-,17+,19-,20+,21+/m1/s1 |
|---|
| InChI Key | RHQQHZQUAMFINJ-DTDWNVJFSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as 21-hydroxysteroids. These are steroids carrying a hydroxyl group at the 21-position of the steroid backbone. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Hydroxysteroids |
|---|
| Direct Parent | 21-hydroxysteroids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Progestogin-skeleton
- 21-hydroxysteroid
- 20-oxosteroid
- Pregnane-skeleton
- 3-hydroxysteroid
- Oxosteroid
- 11-beta-hydroxysteroid
- 11-hydroxysteroid
- 3-alpha-hydroxysteroid
- Cyclic alcohol
- Alpha-hydroxy ketone
- Ketone
- Secondary alcohol
- Alcohol
- Hydrocarbon derivative
- Organooxygen compound
- Organic oxide
- Organic oxygen compound
- Primary alcohol
- Carbonyl group
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | - 21-hydroxy steroid (CHEBI:9481 )
- C21 steroids (gluco/mineralocorticoids, progestogens) and derivatives (C05476 )
- C21 steroids (gluco/mineralocorticoids, progestogins) and derivatives (LMST02030100 )
|
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Not Available |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| Tetrahydrocorticosterone,1TMS,isomer #1 | C[C@]12C[C@H](O)[C@H]3[C@@H](CC[C@@H]4C[C@H](O)CC[C@]34C)[C@@H]1CC[C@@H]2C(=O)CO[Si](C)(C)C | 3123.7 | Semi standard non polar | 33892256 | | Tetrahydrocorticosterone,1TMS,isomer #2 | C[C@]12C[C@H](O[Si](C)(C)C)[C@H]3[C@@H](CC[C@@H]4C[C@H](O)CC[C@]34C)[C@@H]1CC[C@@H]2C(=O)CO | 3062.5 | Semi standard non polar | 33892256 | | Tetrahydrocorticosterone,1TMS,isomer #3 | C[C@]12C[C@H](O)[C@H]3[C@@H](CC[C@@H]4C[C@H](O[Si](C)(C)C)CC[C@]34C)[C@@H]1CC[C@@H]2C(=O)CO | 3126.9 | Semi standard non polar | 33892256 | | Tetrahydrocorticosterone,1TMS,isomer #4 | C[C@]12C[C@H](O)[C@H]3[C@@H](CC[C@@H]4C[C@H](O)CC[C@]34C)[C@@H]1CCC2=C(CO)O[Si](C)(C)C | 3094.7 | Semi standard non polar | 33892256 | | Tetrahydrocorticosterone,1TMS,isomer #5 | C[C@]12C[C@H](O)[C@H]3[C@@H](CC[C@@H]4C[C@H](O)CC[C@]34C)[C@@H]1CC[C@@H]2C(=CO)O[Si](C)(C)C | 3070.6 | Semi standard non polar | 33892256 | | Tetrahydrocorticosterone,2TMS,isomer #1 | C[C@]12C[C@H](O[Si](C)(C)C)[C@H]3[C@@H](CC[C@@H]4C[C@H](O)CC[C@]34C)[C@@H]1CC[C@@H]2C(=O)CO[Si](C)(C)C | 3021.3 | Semi standard non polar | 33892256 | | Tetrahydrocorticosterone,2TMS,isomer #2 | C[C@]12C[C@H](O)[C@H]3[C@@H](CC[C@@H]4C[C@H](O[Si](C)(C)C)CC[C@]34C)[C@@H]1CC[C@@H]2C(=O)CO[Si](C)(C)C | 3103.3 | Semi standard non polar | 33892256 | | Tetrahydrocorticosterone,2TMS,isomer #3 | C[C@]12C[C@H](O)[C@H]3[C@@H](CC[C@@H]4C[C@H](O)CC[C@]34C)[C@@H]1CCC2=C(CO[Si](C)(C)C)O[Si](C)(C)C | 3119.8 | Semi standard non polar | 33892256 | | Tetrahydrocorticosterone,2TMS,isomer #4 | C[C@]12C[C@H](O)[C@H]3[C@@H](CC[C@@H]4C[C@H](O)CC[C@]34C)[C@@H]1CC[C@@H]2C(=CO[Si](C)(C)C)O[Si](C)(C)C | 3101.8 | Semi standard non polar | 33892256 | | Tetrahydrocorticosterone,2TMS,isomer #5 | C[C@]12C[C@H](O[Si](C)(C)C)[C@H]3[C@@H](CC[C@@H]4C[C@H](O[Si](C)(C)C)CC[C@]34C)[C@@H]1CC[C@@H]2C(=O)CO | 3004.3 | Semi standard non polar | 33892256 | | Tetrahydrocorticosterone,2TMS,isomer #6 | C[C@]12C[C@H](O[Si](C)(C)C)[C@H]3[C@@H](CC[C@@H]4C[C@H](O)CC[C@]34C)[C@@H]1CCC2=C(CO)O[Si](C)(C)C | 3018.2 | Semi standard non polar | 33892256 | | Tetrahydrocorticosterone,2TMS,isomer #7 | C[C@]12C[C@H](O[Si](C)(C)C)[C@H]3[C@@H](CC[C@@H]4C[C@H](O)CC[C@]34C)[C@@H]1CC[C@@H]2C(=CO)O[Si](C)(C)C | 2983.2 | Semi standard non polar | 33892256 | | Tetrahydrocorticosterone,2TMS,isomer #8 | C[C@]12C[C@H](O)[C@H]3[C@@H](CC[C@@H]4C[C@H](O[Si](C)(C)C)CC[C@]34C)[C@@H]1CCC2=C(CO)O[Si](C)(C)C | 3106.9 | Semi standard non polar | 33892256 | | Tetrahydrocorticosterone,2TMS,isomer #9 | C[C@]12C[C@H](O)[C@H]3[C@@H](CC[C@@H]4C[C@H](O[Si](C)(C)C)CC[C@]34C)[C@@H]1CC[C@@H]2C(=CO)O[Si](C)(C)C | 3045.2 | Semi standard non polar | 33892256 | | Tetrahydrocorticosterone,3TMS,isomer #1 | C[C@]12C[C@H](O[Si](C)(C)C)[C@H]3[C@@H](CC[C@@H]4C[C@H](O[Si](C)(C)C)CC[C@]34C)[C@@H]1CC[C@@H]2C(=O)CO[Si](C)(C)C | 3001.9 | Semi standard non polar | 33892256 | | Tetrahydrocorticosterone,3TMS,isomer #2 | C[C@]12C[C@H](O[Si](C)(C)C)[C@H]3[C@@H](CC[C@@H]4C[C@H](O)CC[C@]34C)[C@@H]1CCC2=C(CO[Si](C)(C)C)O[Si](C)(C)C | 3017.5 | Semi standard non polar | 33892256 | | Tetrahydrocorticosterone,3TMS,isomer #3 | C[C@]12C[C@H](O[Si](C)(C)C)[C@H]3[C@@H](CC[C@@H]4C[C@H](O)CC[C@]34C)[C@@H]1CC[C@@H]2C(=CO[Si](C)(C)C)O[Si](C)(C)C | 3034.4 | Semi standard non polar | 33892256 | | Tetrahydrocorticosterone,3TMS,isomer #4 | C[C@]12C[C@H](O)[C@H]3[C@@H](CC[C@@H]4C[C@H](O[Si](C)(C)C)CC[C@]34C)[C@@H]1CCC2=C(CO[Si](C)(C)C)O[Si](C)(C)C | 3103.1 | Semi standard non polar | 33892256 | | Tetrahydrocorticosterone,3TMS,isomer #5 | C[C@]12C[C@H](O)[C@H]3[C@@H](CC[C@@H]4C[C@H](O[Si](C)(C)C)CC[C@]34C)[C@@H]1CC[C@@H]2C(=CO[Si](C)(C)C)O[Si](C)(C)C | 3098.0 | Semi standard non polar | 33892256 | | Tetrahydrocorticosterone,3TMS,isomer #6 | C[C@]12C[C@H](O[Si](C)(C)C)[C@H]3[C@@H](CC[C@@H]4C[C@H](O[Si](C)(C)C)CC[C@]34C)[C@@H]1CCC2=C(CO)O[Si](C)(C)C | 3000.3 | Semi standard non polar | 33892256 | | Tetrahydrocorticosterone,3TMS,isomer #7 | C[C@]12C[C@H](O[Si](C)(C)C)[C@H]3[C@@H](CC[C@@H]4C[C@H](O[Si](C)(C)C)CC[C@]34C)[C@@H]1CC[C@@H]2C(=CO)O[Si](C)(C)C | 2965.8 | Semi standard non polar | 33892256 | | Tetrahydrocorticosterone,4TMS,isomer #1 | C[C@]12C[C@H](O[Si](C)(C)C)[C@H]3[C@@H](CC[C@@H]4C[C@H](O[Si](C)(C)C)CC[C@]34C)[C@@H]1CCC2=C(CO[Si](C)(C)C)O[Si](C)(C)C | 3014.0 | Semi standard non polar | 33892256 | | Tetrahydrocorticosterone,4TMS,isomer #1 | C[C@]12C[C@H](O[Si](C)(C)C)[C@H]3[C@@H](CC[C@@H]4C[C@H](O[Si](C)(C)C)CC[C@]34C)[C@@H]1CCC2=C(CO[Si](C)(C)C)O[Si](C)(C)C | 3183.9 | Standard non polar | 33892256 | | Tetrahydrocorticosterone,4TMS,isomer #1 | C[C@]12C[C@H](O[Si](C)(C)C)[C@H]3[C@@H](CC[C@@H]4C[C@H](O[Si](C)(C)C)CC[C@]34C)[C@@H]1CCC2=C(CO[Si](C)(C)C)O[Si](C)(C)C | 3346.4 | Standard polar | 33892256 | | Tetrahydrocorticosterone,4TMS,isomer #2 | C[C@]12C[C@H](O[Si](C)(C)C)[C@H]3[C@@H](CC[C@@H]4C[C@H](O[Si](C)(C)C)CC[C@]34C)[C@@H]1CC[C@@H]2C(=CO[Si](C)(C)C)O[Si](C)(C)C | 3057.7 | Semi standard non polar | 33892256 | | Tetrahydrocorticosterone,4TMS,isomer #2 | C[C@]12C[C@H](O[Si](C)(C)C)[C@H]3[C@@H](CC[C@@H]4C[C@H](O[Si](C)(C)C)CC[C@]34C)[C@@H]1CC[C@@H]2C(=CO[Si](C)(C)C)O[Si](C)(C)C | 3083.5 | Standard non polar | 33892256 | | Tetrahydrocorticosterone,4TMS,isomer #2 | C[C@]12C[C@H](O[Si](C)(C)C)[C@H]3[C@@H](CC[C@@H]4C[C@H](O[Si](C)(C)C)CC[C@]34C)[C@@H]1CC[C@@H]2C(=CO[Si](C)(C)C)O[Si](C)(C)C | 3401.1 | Standard polar | 33892256 | | Tetrahydrocorticosterone,1TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OCC(=O)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3[C@@H](O)C[C@]12C | 3416.3 | Semi standard non polar | 33892256 | | Tetrahydrocorticosterone,1TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)O[C@H]1C[C@]2(C)[C@@H](C(=O)CO)CC[C@H]2[C@@H]2CC[C@@H]3C[C@H](O)CC[C@]3(C)[C@@H]12 | 3315.1 | Semi standard non polar | 33892256 | | Tetrahydrocorticosterone,1TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)O[C@@H]1CC[C@]2(C)[C@H]3[C@@H](O)C[C@]4(C)[C@@H](C(=O)CO)CC[C@H]4[C@@H]3CC[C@@H]2C1 | 3378.7 | Semi standard non polar | 33892256 | | Tetrahydrocorticosterone,1TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)OC(CO)=C1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3[C@@H](O)C[C@]12C | 3382.5 | Semi standard non polar | 33892256 | | Tetrahydrocorticosterone,1TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)OC(=CO)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3[C@@H](O)C[C@]12C | 3319.3 | Semi standard non polar | 33892256 | | Tetrahydrocorticosterone,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OCC(=O)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O[Si](C)(C)C(C)(C)C)CC[C@]4(C)[C@H]3[C@@H](O)C[C@]12C | 3620.9 | Semi standard non polar | 33892256 | | Tetrahydrocorticosterone,2TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OCC(=O)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3[C@@H](O[Si](C)(C)C(C)(C)C)C[C@]12C | 3520.1 | Semi standard non polar | 33892256 | | Tetrahydrocorticosterone,2TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OCC(O[Si](C)(C)C(C)(C)C)=C1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3[C@@H](O)C[C@]12C | 3652.6 | Semi standard non polar | 33892256 | | Tetrahydrocorticosterone,2TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)OC=C(O[Si](C)(C)C(C)(C)C)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3[C@@H](O)C[C@]12C | 3565.2 | Semi standard non polar | 33892256 | | Tetrahydrocorticosterone,2TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)O[C@H]1C[C@]2(C)[C@@H](C(=O)CO)CC[C@H]2[C@@H]2CC[C@@H]3C[C@H](O[Si](C)(C)C(C)(C)C)CC[C@]3(C)[C@@H]12 | 3460.8 | Semi standard non polar | 33892256 | | Tetrahydrocorticosterone,2TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)OC(CO)=C1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3[C@@H](O[Si](C)(C)C(C)(C)C)C[C@]12C | 3501.1 | Semi standard non polar | 33892256 | | Tetrahydrocorticosterone,2TBDMS,isomer #7 | CC(C)(C)[Si](C)(C)OC(=CO)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3[C@@H](O[Si](C)(C)C(C)(C)C)C[C@]12C | 3423.5 | Semi standard non polar | 33892256 | | Tetrahydrocorticosterone,2TBDMS,isomer #8 | CC(C)(C)[Si](C)(C)OC(CO)=C1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O[Si](C)(C)C(C)(C)C)CC[C@]4(C)[C@H]3[C@@H](O)C[C@]12C | 3620.1 | Semi standard non polar | 33892256 | | Tetrahydrocorticosterone,2TBDMS,isomer #9 | CC(C)(C)[Si](C)(C)OC(=CO)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O[Si](C)(C)C(C)(C)C)CC[C@]4(C)[C@H]3[C@@H](O)C[C@]12C | 3524.3 | Semi standard non polar | 33892256 | | Tetrahydrocorticosterone,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OCC(=O)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O[Si](C)(C)C(C)(C)C)CC[C@]4(C)[C@H]3[C@@H](O[Si](C)(C)C(C)(C)C)C[C@]12C | 3686.1 | Semi standard non polar | 33892256 | | Tetrahydrocorticosterone,3TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OCC(O[Si](C)(C)C(C)(C)C)=C1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O[Si](C)(C)C(C)(C)C)CC[C@]4(C)[C@H]3[C@@H](O)C[C@]12C | 3847.6 | Semi standard non polar | 33892256 | | Tetrahydrocorticosterone,3TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC=C(O[Si](C)(C)C(C)(C)C)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O[Si](C)(C)C(C)(C)C)CC[C@]4(C)[C@H]3[C@@H](O)C[C@]12C | 3771.0 | Semi standard non polar | 33892256 | | Tetrahydrocorticosterone,3TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)OCC(O[Si](C)(C)C(C)(C)C)=C1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3[C@@H](O[Si](C)(C)C(C)(C)C)C[C@]12C | 3690.8 | Semi standard non polar | 33892256 | | Tetrahydrocorticosterone,3TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)OC=C(O[Si](C)(C)C(C)(C)C)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3[C@@H](O[Si](C)(C)C(C)(C)C)C[C@]12C | 3678.8 | Semi standard non polar | 33892256 | | Tetrahydrocorticosterone,3TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)OC(CO)=C1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O[Si](C)(C)C(C)(C)C)CC[C@]4(C)[C@H]3[C@@H](O[Si](C)(C)C(C)(C)C)C[C@]12C | 3663.3 | Semi standard non polar | 33892256 | | Tetrahydrocorticosterone,3TBDMS,isomer #7 | CC(C)(C)[Si](C)(C)OC(=CO)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O[Si](C)(C)C(C)(C)C)CC[C@]4(C)[C@H]3[C@@H](O[Si](C)(C)C(C)(C)C)C[C@]12C | 3618.6 | Semi standard non polar | 33892256 | | Tetrahydrocorticosterone,4TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OCC(O[Si](C)(C)C(C)(C)C)=C1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O[Si](C)(C)C(C)(C)C)CC[C@]4(C)[C@H]3[C@@H](O[Si](C)(C)C(C)(C)C)C[C@]12C | 3851.8 | Semi standard non polar | 33892256 | | Tetrahydrocorticosterone,4TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OCC(O[Si](C)(C)C(C)(C)C)=C1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O[Si](C)(C)C(C)(C)C)CC[C@]4(C)[C@H]3[C@@H](O[Si](C)(C)C(C)(C)C)C[C@]12C | 3984.0 | Standard non polar | 33892256 | | Tetrahydrocorticosterone,4TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OCC(O[Si](C)(C)C(C)(C)C)=C1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O[Si](C)(C)C(C)(C)C)CC[C@]4(C)[C@H]3[C@@H](O[Si](C)(C)C(C)(C)C)C[C@]12C | 3664.3 | Standard polar | 33892256 | | Tetrahydrocorticosterone,4TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OC=C(O[Si](C)(C)C(C)(C)C)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O[Si](C)(C)C(C)(C)C)CC[C@]4(C)[C@H]3[C@@H](O[Si](C)(C)C(C)(C)C)C[C@]12C | 3882.1 | Semi standard non polar | 33892256 | | Tetrahydrocorticosterone,4TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OC=C(O[Si](C)(C)C(C)(C)C)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O[Si](C)(C)C(C)(C)C)CC[C@]4(C)[C@H]3[C@@H](O[Si](C)(C)C(C)(C)C)C[C@]12C | 3858.9 | Standard non polar | 33892256 | | Tetrahydrocorticosterone,4TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OC=C(O[Si](C)(C)C(C)(C)C)[C@H]1CC[C@H]2[C@@H]3CC[C@@H]4C[C@H](O[Si](C)(C)C(C)(C)C)CC[C@]4(C)[C@H]3[C@@H](O[Si](C)(C)C(C)(C)C)C[C@]12C | 3680.6 | Standard polar | 33892256 |
|
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - Tetrahydrocorticosterone GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Tetrahydrocorticosterone GC-MS (TMS_1_1) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Tetrahydrocorticosterone GC-MS (TMS_1_2) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Tetrahydrocorticosterone GC-MS (TMS_1_3) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Tetrahydrocorticosterone GC-MS (TMS_1_4) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Tetrahydrocorticosterone GC-MS (TMS_1_5) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Tetrahydrocorticosterone GC-MS (TMS_2_1) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Tetrahydrocorticosterone GC-MS (TMS_2_2) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Tetrahydrocorticosterone GC-MS (TMS_2_3) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Tetrahydrocorticosterone GC-MS (TMS_2_4) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Tetrahydrocorticosterone GC-MS (TMS_2_5) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Tetrahydrocorticosterone GC-MS (TMS_2_6) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Tetrahydrocorticosterone GC-MS (TMS_2_7) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Tetrahydrocorticosterone GC-MS (TMS_2_8) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Tetrahydrocorticosterone GC-MS (TMS_2_9) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Tetrahydrocorticosterone GC-MS (TMS_3_1) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Tetrahydrocorticosterone GC-MS (TMS_3_2) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Tetrahydrocorticosterone GC-MS (TMS_3_3) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Tetrahydrocorticosterone GC-MS (TMS_3_4) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Tetrahydrocorticosterone GC-MS (TMS_3_5) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Tetrahydrocorticosterone GC-MS (TMS_3_6) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Tetrahydrocorticosterone GC-MS (TMS_3_7) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Tetrahydrocorticosterone GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Tetrahydrocorticosterone GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Tetrahydrocorticosterone GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental LC-MS/MS | LC-MS/MS Spectrum - Tetrahydrocorticosterone Quattro_QQQ 10V, Positive-QTOF (Annotated) | splash10-0002-0009000000-cec330110dfc2d03e126 | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Tetrahydrocorticosterone Quattro_QQQ 25V, Positive-QTOF (Annotated) | splash10-0592-9077000000-7adca5a0af60b9fda618 | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Tetrahydrocorticosterone Quattro_QQQ 40V, Positive-QTOF (Annotated) | splash10-0udi-3490000000-84176a53f54507ead0c6 | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Tetrahydrocorticosterone EI-B (HITACHI M-80) , Positive-QTOF | splash10-01b9-1947000000-cbb72686416e9b705c28 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Tetrahydrocorticosterone 10V, Positive-QTOF | splash10-0fsi-0019000000-3e07505796a0d0ccf759 | 2019-02-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Tetrahydrocorticosterone 20V, Positive-QTOF | splash10-0159-0029000000-a684771622b2b677b05e | 2019-02-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Tetrahydrocorticosterone 40V, Positive-QTOF | splash10-0fmi-3193000000-258bcce49fc0fdfa367c | 2019-02-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Tetrahydrocorticosterone 10V, Negative-QTOF | splash10-0002-0009000000-604ddf044ec2555026ca | 2019-02-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Tetrahydrocorticosterone 20V, Negative-QTOF | splash10-05o1-1029000000-5f7d67b696b8216549c8 | 2019-02-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Tetrahydrocorticosterone 40V, Negative-QTOF | splash10-0c2c-4094000000-6414e1ce977710e5f136 | 2019-02-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Tetrahydrocorticosterone 10V, Positive-QTOF | splash10-0ue9-0009000000-0aa7b6bcf5326ebfd06d | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Tetrahydrocorticosterone 20V, Positive-QTOF | splash10-0g59-1946000000-70f8a3a83793bdccde86 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Tetrahydrocorticosterone 40V, Positive-QTOF | splash10-014l-9360000000-b75838c5554768281848 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Tetrahydrocorticosterone 10V, Negative-QTOF | splash10-0002-0009000000-ce9bde525139d21c8d92 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Tetrahydrocorticosterone 20V, Negative-QTOF | splash10-00kb-0009000000-3c63ef8c1c108dd51eec | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Tetrahydrocorticosterone 40V, Negative-QTOF | splash10-0hg2-1049000000-d6e8731409c9d6502763 | 2021-09-22 | Wishart Lab | View Spectrum |
|
|---|