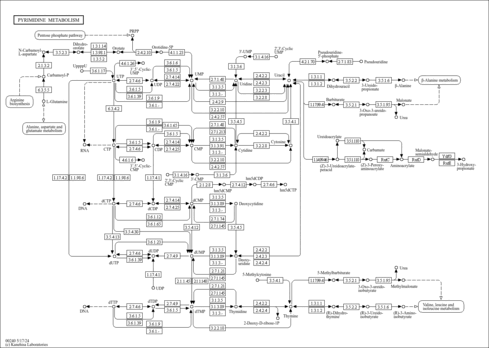
| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| Uridine triphosphate,1TMS,isomer #1 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O)O)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O | 3653.8 | Semi standard non polar | 33892256 |
| Uridine triphosphate,1TMS,isomer #2 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O)O)O[C@H]1N1C=CC(=O)[NH]C1=O | 3648.0 | Semi standard non polar | 33892256 |
| Uridine triphosphate,1TMS,isomer #3 | C[Si](C)(C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(=O)[NH]C2=O)[C@H](O)[C@@H]1O)OP(=O)(O)OP(=O)(O)O | 3725.8 | Semi standard non polar | 33892256 |
| Uridine triphosphate,1TMS,isomer #4 | C[Si](C)(C)OP(=O)(OP(=O)(O)O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(=O)[NH]C2=O)[C@H](O)[C@@H]1O | 3737.0 | Semi standard non polar | 33892256 |
| Uridine triphosphate,1TMS,isomer #5 | C[Si](C)(C)OP(=O)(O)OP(=O)(O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(=O)[NH]C2=O)[C@H](O)[C@@H]1O | 3712.0 | Semi standard non polar | 33892256 |
| Uridine triphosphate,1TMS,isomer #6 | C[Si](C)(C)N1C(=O)C=CN([C@@H]2O[C@H](COP(=O)(O)OP(=O)(O)OP(=O)(O)O)[C@@H](O)[C@H]2O)C1=O | 3748.2 | Semi standard non polar | 33892256 |
| Uridine triphosphate,2TMS,isomer #1 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O)O)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O[Si](C)(C)C | 3506.9 | Semi standard non polar | 33892256 |
| Uridine triphosphate,2TMS,isomer #10 | C[Si](C)(C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(=O)[NH]C2=O)[C@H](O)[C@@H]1O)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O | 3659.6 | Semi standard non polar | 33892256 |
| Uridine triphosphate,2TMS,isomer #11 | C[Si](C)(C)OP(=O)(O)OP(=O)(O)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(=O)[NH]C2=O)[C@H](O)[C@@H]1O)O[Si](C)(C)C | 3652.0 | Semi standard non polar | 33892256 |
| Uridine triphosphate,2TMS,isomer #12 | C[Si](C)(C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@H](O)[C@@H]1O)OP(=O)(O)OP(=O)(O)O | 3702.8 | Semi standard non polar | 33892256 |
| Uridine triphosphate,2TMS,isomer #13 | C[Si](C)(C)OP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(=O)[NH]C2=O)[C@H](O)[C@@H]1O | 3647.9 | Semi standard non polar | 33892256 |
| Uridine triphosphate,2TMS,isomer #14 | C[Si](C)(C)OP(=O)(OP(=O)(O)O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@H](O)[C@@H]1O | 3717.6 | Semi standard non polar | 33892256 |
| Uridine triphosphate,2TMS,isomer #15 | C[Si](C)(C)OP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(=O)[NH]C2=O)[C@H](O)[C@@H]1O | 3652.4 | Semi standard non polar | 33892256 |
| Uridine triphosphate,2TMS,isomer #16 | C[Si](C)(C)OP(=O)(O)OP(=O)(O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@H](O)[C@@H]1O | 3693.9 | Semi standard non polar | 33892256 |
| Uridine triphosphate,2TMS,isomer #2 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O)O)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O | 3586.2 | Semi standard non polar | 33892256 |
| Uridine triphosphate,2TMS,isomer #3 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O | 3589.0 | Semi standard non polar | 33892256 |
| Uridine triphosphate,2TMS,isomer #4 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O | 3573.8 | Semi standard non polar | 33892256 |
| Uridine triphosphate,2TMS,isomer #5 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O)O)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O | 3632.2 | Semi standard non polar | 33892256 |
| Uridine triphosphate,2TMS,isomer #6 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O)O)O[C@H]1N1C=CC(=O)[NH]C1=O | 3569.8 | Semi standard non polar | 33892256 |
| Uridine triphosphate,2TMS,isomer #7 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O)O[C@H]1N1C=CC(=O)[NH]C1=O | 3575.2 | Semi standard non polar | 33892256 |
| Uridine triphosphate,2TMS,isomer #8 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O)O[Si](C)(C)C)O[C@H]1N1C=CC(=O)[NH]C1=O | 3564.2 | Semi standard non polar | 33892256 |
| Uridine triphosphate,2TMS,isomer #9 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O)O)O[C@H]1N1C=CC(=O)N([Si](C)(C)C)C1=O | 3623.7 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #1 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O)O)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O[Si](C)(C)C | 3476.7 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #1 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O)O)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O[Si](C)(C)C | 3615.1 | Standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #1 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O)O)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O[Si](C)(C)C | 5345.5 | Standard polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #10 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O | 3546.4 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #10 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O | 3655.0 | Standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #10 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O | 5198.4 | Standard polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #11 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O | 3568.4 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #11 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O | 3686.2 | Standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #11 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O | 5507.3 | Standard polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #12 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O)O[C@H]1N1C=CC(=O)[NH]C1=O | 3524.6 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #12 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O)O[C@H]1N1C=CC(=O)[NH]C1=O | 3712.1 | Standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #12 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O)O[C@H]1N1C=CC(=O)[NH]C1=O | 5092.8 | Standard polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #13 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O)O[Si](C)(C)C)O[C@H]1N1C=CC(=O)[NH]C1=O | 3537.2 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #13 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O)O[Si](C)(C)C)O[C@H]1N1C=CC(=O)[NH]C1=O | 3692.0 | Standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #13 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O)O[Si](C)(C)C)O[C@H]1N1C=CC(=O)[NH]C1=O | 5199.0 | Standard polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #14 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O)O)O[C@H]1N1C=CC(=O)N([Si](C)(C)C)C1=O | 3574.1 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #14 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O)O)O[C@H]1N1C=CC(=O)N([Si](C)(C)C)C1=O | 3705.5 | Standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #14 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O)O)O[C@H]1N1C=CC(=O)N([Si](C)(C)C)C1=O | 5467.2 | Standard polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #15 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O[Si](C)(C)C)O[C@H]1N1C=CC(=O)[NH]C1=O | 3532.8 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #15 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O[Si](C)(C)C)O[C@H]1N1C=CC(=O)[NH]C1=O | 3703.9 | Standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #15 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O[Si](C)(C)C)O[C@H]1N1C=CC(=O)[NH]C1=O | 5157.8 | Standard polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #16 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O)O[C@H]1N1C=CC(=O)N([Si](C)(C)C)C1=O | 3573.5 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #16 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O)O[C@H]1N1C=CC(=O)N([Si](C)(C)C)C1=O | 3713.6 | Standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #16 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O)O[C@H]1N1C=CC(=O)N([Si](C)(C)C)C1=O | 5422.4 | Standard polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #17 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@H]1N1C=CC(=O)[NH]C1=O | 3540.3 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #17 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@H]1N1C=CC(=O)[NH]C1=O | 3682.3 | Standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #17 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@H]1N1C=CC(=O)[NH]C1=O | 5215.4 | Standard polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #18 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O)O[Si](C)(C)C)O[C@H]1N1C=CC(=O)N([Si](C)(C)C)C1=O | 3563.3 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #18 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O)O[Si](C)(C)C)O[C@H]1N1C=CC(=O)N([Si](C)(C)C)C1=O | 3694.8 | Standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #18 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O)O[Si](C)(C)C)O[C@H]1N1C=CC(=O)N([Si](C)(C)C)C1=O | 5523.4 | Standard polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #19 | C[Si](C)(C)OP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(=O)[NH]C2=O)[C@H](O)[C@@H]1O)O[Si](C)(C)C | 3589.4 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #19 | C[Si](C)(C)OP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(=O)[NH]C2=O)[C@H](O)[C@@H]1O)O[Si](C)(C)C | 3770.2 | Standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #19 | C[Si](C)(C)OP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(=O)[NH]C2=O)[C@H](O)[C@@H]1O)O[Si](C)(C)C | 4971.3 | Standard polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #2 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O[Si](C)(C)C | 3488.7 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #2 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O[Si](C)(C)C | 3618.5 | Standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #2 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O[Si](C)(C)C | 5308.1 | Standard polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #20 | C[Si](C)(C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@H](O)[C@@H]1O)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O | 3638.7 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #20 | C[Si](C)(C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@H](O)[C@@H]1O)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O | 3789.9 | Standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #20 | C[Si](C)(C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@H](O)[C@@H]1O)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O | 5214.5 | Standard polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #21 | C[Si](C)(C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(=O)[NH]C2=O)[C@H](O)[C@@H]1O)OP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 3612.6 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #21 | C[Si](C)(C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(=O)[NH]C2=O)[C@H](O)[C@@H]1O)OP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 3746.3 | Standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #21 | C[Si](C)(C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(=O)[NH]C2=O)[C@H](O)[C@@H]1O)OP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 5003.9 | Standard polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #22 | C[Si](C)(C)OP(=O)(O)OP(=O)(O)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@H](O)[C@@H]1O)O[Si](C)(C)C | 3632.3 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #22 | C[Si](C)(C)OP(=O)(O)OP(=O)(O)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@H](O)[C@@H]1O)O[Si](C)(C)C | 3765.3 | Standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #22 | C[Si](C)(C)OP(=O)(O)OP(=O)(O)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@H](O)[C@@H]1O)O[Si](C)(C)C | 5317.7 | Standard polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #23 | C[Si](C)(C)OP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(=O)[NH]C2=O)[C@H](O)[C@@H]1O | 3598.5 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #23 | C[Si](C)(C)OP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(=O)[NH]C2=O)[C@H](O)[C@@H]1O | 3755.1 | Standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #23 | C[Si](C)(C)OP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(=O)[NH]C2=O)[C@H](O)[C@@H]1O | 4991.7 | Standard polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #24 | C[Si](C)(C)OP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@H](O)[C@@H]1O | 3627.5 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #24 | C[Si](C)(C)OP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@H](O)[C@@H]1O | 3783.4 | Standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #24 | C[Si](C)(C)OP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@H](O)[C@@H]1O | 5280.3 | Standard polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #25 | C[Si](C)(C)OP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@H](O)[C@@H]1O | 3634.7 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #25 | C[Si](C)(C)OP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@H](O)[C@@H]1O | 3757.8 | Standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #25 | C[Si](C)(C)OP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@H](O)[C@@H]1O | 5339.2 | Standard polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #3 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O[Si](C)(C)C | 3483.6 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #3 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O[Si](C)(C)C | 3602.4 | Standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #3 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O[Si](C)(C)C | 5413.0 | Standard polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #4 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O)O)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O[Si](C)(C)C | 3537.6 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #4 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O)O)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O[Si](C)(C)C | 3629.5 | Standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #4 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O)O)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O[Si](C)(C)C | 5704.5 | Standard polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #5 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O | 3533.8 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #5 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O | 3685.1 | Standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #5 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O | 5066.0 | Standard polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #6 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O | 3540.7 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #6 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O | 3663.4 | Standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #6 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O | 5173.2 | Standard polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #7 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O)O)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O | 3580.1 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #7 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O)O)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O | 3697.6 | Standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #7 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O)O)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O | 5441.0 | Standard polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #8 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O | 3538.7 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #8 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O | 3677.0 | Standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #8 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O | 5140.2 | Standard polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #9 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O | 3580.2 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #9 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O | 3706.8 | Standard non polar | 33892256 |
| Uridine triphosphate,3TMS,isomer #9 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O | 5405.5 | Standard polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #1 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O[Si](C)(C)C | 3474.2 | Semi standard non polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #1 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O[Si](C)(C)C | 3635.2 | Standard non polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #1 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O[Si](C)(C)C | 4858.2 | Standard polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #10 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O | 3557.9 | Semi standard non polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #10 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O | 3642.8 | Standard non polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #10 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O | 4779.3 | Standard polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #11 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O | 3559.0 | Semi standard non polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #11 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O | 3675.9 | Standard non polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #11 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O | 5034.6 | Standard polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #12 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O | 3551.5 | Semi standard non polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #12 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O | 3649.6 | Standard non polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #12 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O | 4752.8 | Standard polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #13 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O | 3553.1 | Semi standard non polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #13 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O | 3687.9 | Standard non polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #13 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O | 4976.5 | Standard polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #14 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O | 3563.8 | Semi standard non polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #14 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O | 3667.9 | Standard non polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #14 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O | 5062.2 | Standard polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #15 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O[Si](C)(C)C)O[C@H]1N1C=CC(=O)[NH]C1=O | 3539.9 | Semi standard non polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #15 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O[Si](C)(C)C)O[C@H]1N1C=CC(=O)[NH]C1=O | 3699.9 | Standard non polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #15 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O[Si](C)(C)C)O[C@H]1N1C=CC(=O)[NH]C1=O | 4750.6 | Standard polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #16 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O)O[C@H]1N1C=CC(=O)N([Si](C)(C)C)C1=O | 3547.2 | Semi standard non polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #16 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O)O[C@H]1N1C=CC(=O)N([Si](C)(C)C)C1=O | 3705.8 | Standard non polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #16 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O)O[C@H]1N1C=CC(=O)N([Si](C)(C)C)C1=O | 4941.3 | Standard polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #17 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@H]1N1C=CC(=O)[NH]C1=O | 3551.7 | Semi standard non polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #17 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@H]1N1C=CC(=O)[NH]C1=O | 3673.9 | Standard non polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #17 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@H]1N1C=CC(=O)[NH]C1=O | 4807.6 | Standard polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #18 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O)O[Si](C)(C)C)O[C@H]1N1C=CC(=O)N([Si](C)(C)C)C1=O | 3559.2 | Semi standard non polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #18 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O)O[Si](C)(C)C)O[C@H]1N1C=CC(=O)N([Si](C)(C)C)C1=O | 3682.8 | Standard non polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #18 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O)O[Si](C)(C)C)O[C@H]1N1C=CC(=O)N([Si](C)(C)C)C1=O | 5062.1 | Standard polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #19 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@H]1N1C=CC(=O)[NH]C1=O | 3543.8 | Semi standard non polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #19 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@H]1N1C=CC(=O)[NH]C1=O | 3679.6 | Standard non polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #19 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@H]1N1C=CC(=O)[NH]C1=O | 4768.6 | Standard polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #2 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O[Si](C)(C)C | 3492.8 | Semi standard non polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #2 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O[Si](C)(C)C | 3616.4 | Standard non polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #2 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O[Si](C)(C)C | 4980.9 | Standard polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #20 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O[Si](C)(C)C)O[C@H]1N1C=CC(=O)N([Si](C)(C)C)C1=O | 3551.9 | Semi standard non polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #20 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O[Si](C)(C)C)O[C@H]1N1C=CC(=O)N([Si](C)(C)C)C1=O | 3693.3 | Standard non polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #20 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O[Si](C)(C)C)O[C@H]1N1C=CC(=O)N([Si](C)(C)C)C1=O | 4993.5 | Standard polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #21 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@H]1N1C=CC(=O)N([Si](C)(C)C)C1=O | 3561.7 | Semi standard non polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #21 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@H]1N1C=CC(=O)N([Si](C)(C)C)C1=O | 3673.7 | Standard non polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #21 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@H]1N1C=CC(=O)N([Si](C)(C)C)C1=O | 5079.5 | Standard polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #22 | C[Si](C)(C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(=O)[NH]C2=O)[C@H](O)[C@@H]1O)OP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 3603.2 | Semi standard non polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #22 | C[Si](C)(C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(=O)[NH]C2=O)[C@H](O)[C@@H]1O)OP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 3741.7 | Standard non polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #22 | C[Si](C)(C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(=O)[NH]C2=O)[C@H](O)[C@@H]1O)OP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 4573.4 | Standard polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #23 | C[Si](C)(C)OP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@H](O)[C@@H]1O)O[Si](C)(C)C | 3599.0 | Semi standard non polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #23 | C[Si](C)(C)OP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@H](O)[C@@H]1O)O[Si](C)(C)C | 3759.0 | Standard non polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #23 | C[Si](C)(C)OP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@H](O)[C@@H]1O)O[Si](C)(C)C | 4828.8 | Standard polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #24 | C[Si](C)(C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@H](O)[C@@H]1O)OP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 3623.3 | Semi standard non polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #24 | C[Si](C)(C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@H](O)[C@@H]1O)OP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 3733.3 | Standard non polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #24 | C[Si](C)(C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@H](O)[C@@H]1O)OP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 4886.1 | Standard polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #25 | C[Si](C)(C)OP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@H](O)[C@@H]1O | 3611.4 | Semi standard non polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #25 | C[Si](C)(C)OP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@H](O)[C@@H]1O | 3741.3 | Standard non polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #25 | C[Si](C)(C)OP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@H](O)[C@@H]1O | 4854.3 | Standard polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #3 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O)O)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O[Si](C)(C)C | 3522.6 | Semi standard non polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #3 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O)O)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O[Si](C)(C)C | 3637.2 | Standard non polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #3 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O)O)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O[Si](C)(C)C | 5185.3 | Standard polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #4 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O[Si](C)(C)C | 3491.6 | Semi standard non polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #4 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O[Si](C)(C)C | 3622.7 | Standard non polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #4 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O[Si](C)(C)C | 4923.4 | Standard polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #5 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O[Si](C)(C)C | 3519.5 | Semi standard non polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #5 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O[Si](C)(C)C | 3639.6 | Standard non polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #5 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O[Si](C)(C)C | 5125.0 | Standard polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #6 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O[Si](C)(C)C | 3489.4 | Semi standard non polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #6 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O[Si](C)(C)C | 3604.6 | Standard non polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #6 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O[Si](C)(C)C | 5004.9 | Standard polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #7 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O[Si](C)(C)C | 3523.2 | Semi standard non polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #7 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O[Si](C)(C)C | 3627.2 | Standard non polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #7 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O[Si](C)(C)C | 5242.8 | Standard polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #8 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O | 3547.2 | Semi standard non polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #8 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O | 3668.4 | Standard non polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #8 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O | 4722.7 | Standard polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #9 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O | 3550.5 | Semi standard non polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #9 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O | 3699.1 | Standard non polar | 33892256 |
| Uridine triphosphate,4TMS,isomer #9 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O | 4913.5 | Standard polar | 33892256 |
| Uridine triphosphate,5TMS,isomer #1 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O[Si](C)(C)C | 3502.1 | Semi standard non polar | 33892256 |
| Uridine triphosphate,5TMS,isomer #1 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O[Si](C)(C)C | 3608.5 | Standard non polar | 33892256 |
| Uridine triphosphate,5TMS,isomer #1 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O[Si](C)(C)C | 4520.9 | Standard polar | 33892256 |
| Uridine triphosphate,5TMS,isomer #10 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O | 3617.7 | Semi standard non polar | 33892256 |
| Uridine triphosphate,5TMS,isomer #10 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O | 3641.6 | Standard non polar | 33892256 |
| Uridine triphosphate,5TMS,isomer #10 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O | 4621.9 | Standard polar | 33892256 |
| Uridine triphosphate,5TMS,isomer #11 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O | 3602.6 | Semi standard non polar | 33892256 |
| Uridine triphosphate,5TMS,isomer #11 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O | 3650.1 | Standard non polar | 33892256 |
| Uridine triphosphate,5TMS,isomer #11 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O | 4574.1 | Standard polar | 33892256 |
| Uridine triphosphate,5TMS,isomer #12 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@H]1N1C=CC(=O)[NH]C1=O | 3549.3 | Semi standard non polar | 33892256 |
| Uridine triphosphate,5TMS,isomer #12 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@H]1N1C=CC(=O)[NH]C1=O | 3653.9 | Standard non polar | 33892256 |
| Uridine triphosphate,5TMS,isomer #12 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@H]1N1C=CC(=O)[NH]C1=O | 4347.6 | Standard polar | 33892256 |
| Uridine triphosphate,5TMS,isomer #13 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O[Si](C)(C)C)O[C@H]1N1C=CC(=O)N([Si](C)(C)C)C1=O | 3583.0 | Semi standard non polar | 33892256 |
| Uridine triphosphate,5TMS,isomer #13 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O[Si](C)(C)C)O[C@H]1N1C=CC(=O)N([Si](C)(C)C)C1=O | 3676.4 | Standard non polar | 33892256 |
| Uridine triphosphate,5TMS,isomer #13 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O[Si](C)(C)C)O[C@H]1N1C=CC(=O)N([Si](C)(C)C)C1=O | 4573.9 | Standard polar | 33892256 |
| Uridine triphosphate,5TMS,isomer #14 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@H]1N1C=CC(=O)N([Si](C)(C)C)C1=O | 3606.4 | Semi standard non polar | 33892256 |
| Uridine triphosphate,5TMS,isomer #14 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@H]1N1C=CC(=O)N([Si](C)(C)C)C1=O | 3650.6 | Standard non polar | 33892256 |
| Uridine triphosphate,5TMS,isomer #14 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@H]1N1C=CC(=O)N([Si](C)(C)C)C1=O | 4650.6 | Standard polar | 33892256 |
| Uridine triphosphate,5TMS,isomer #15 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@H]1N1C=CC(=O)N([Si](C)(C)C)C1=O | 3591.7 | Semi standard non polar | 33892256 |
| Uridine triphosphate,5TMS,isomer #15 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@H]1N1C=CC(=O)N([Si](C)(C)C)C1=O | 3658.6 | Standard non polar | 33892256 |
| Uridine triphosphate,5TMS,isomer #15 | C[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@H]1N1C=CC(=O)N([Si](C)(C)C)C1=O | 4591.0 | Standard polar | 33892256 |
| Uridine triphosphate,5TMS,isomer #16 | C[Si](C)(C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@H](O)[C@@H]1O)OP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 3637.0 | Semi standard non polar | 33892256 |
| Uridine triphosphate,5TMS,isomer #16 | C[Si](C)(C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@H](O)[C@@H]1O)OP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 3705.7 | Standard non polar | 33892256 |
| Uridine triphosphate,5TMS,isomer #16 | C[Si](C)(C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@H](O)[C@@H]1O)OP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 4402.4 | Standard polar | 33892256 |
| Uridine triphosphate,5TMS,isomer #2 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O[Si](C)(C)C | 3549.0 | Semi standard non polar | 33892256 |
| Uridine triphosphate,5TMS,isomer #2 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O[Si](C)(C)C | 3630.1 | Standard non polar | 33892256 |
| Uridine triphosphate,5TMS,isomer #2 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O[Si](C)(C)C | 4683.6 | Standard polar | 33892256 |
| Uridine triphosphate,5TMS,isomer #3 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O[Si](C)(C)C | 3508.5 | Semi standard non polar | 33892256 |
| Uridine triphosphate,5TMS,isomer #3 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O[Si](C)(C)C | 3584.1 | Standard non polar | 33892256 |
| Uridine triphosphate,5TMS,isomer #3 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O[Si](C)(C)C | 4586.7 | Standard polar | 33892256 |
| Uridine triphosphate,5TMS,isomer #4 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O[Si](C)(C)C | 3563.6 | Semi standard non polar | 33892256 |
| Uridine triphosphate,5TMS,isomer #4 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O[Si](C)(C)C | 3614.0 | Standard non polar | 33892256 |
| Uridine triphosphate,5TMS,isomer #4 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O)OP(=O)(O)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O[Si](C)(C)C | 4830.9 | Standard polar | 33892256 |
| Uridine triphosphate,5TMS,isomer #5 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O[Si](C)(C)C | 3496.1 | Semi standard non polar | 33892256 |
| Uridine triphosphate,5TMS,isomer #5 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O[Si](C)(C)C | 3592.5 | Standard non polar | 33892256 |
| Uridine triphosphate,5TMS,isomer #5 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O[Si](C)(C)C | 4542.6 | Standard polar | 33892256 |
| Uridine triphosphate,5TMS,isomer #6 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O[Si](C)(C)C | 3554.7 | Semi standard non polar | 33892256 |
| Uridine triphosphate,5TMS,isomer #6 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O[Si](C)(C)C | 3624.5 | Standard non polar | 33892256 |
| Uridine triphosphate,5TMS,isomer #6 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O[Si](C)(C)C | 4740.4 | Standard polar | 33892256 |
| Uridine triphosphate,5TMS,isomer #7 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O[Si](C)(C)C | 3562.6 | Semi standard non polar | 33892256 |
| Uridine triphosphate,5TMS,isomer #7 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O[Si](C)(C)C | 3606.9 | Standard non polar | 33892256 |
| Uridine triphosphate,5TMS,isomer #7 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O[Si](C)(C)C | 4860.0 | Standard polar | 33892256 |
| Uridine triphosphate,5TMS,isomer #8 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O | 3559.8 | Semi standard non polar | 33892256 |
| Uridine triphosphate,5TMS,isomer #8 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O | 3623.8 | Standard non polar | 33892256 |
| Uridine triphosphate,5TMS,isomer #8 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O | 4315.6 | Standard polar | 33892256 |
| Uridine triphosphate,5TMS,isomer #9 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O | 3595.3 | Semi standard non polar | 33892256 |
| Uridine triphosphate,5TMS,isomer #9 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O | 3667.5 | Standard non polar | 33892256 |
| Uridine triphosphate,5TMS,isomer #9 | C[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)OP(=O)(O)O[Si](C)(C)C)O[C@@H](N2C=CC(=O)N([Si](C)(C)C)C2=O)[C@@H]1O | 4544.3 | Standard polar | 33892256 |
| Uridine triphosphate,1TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O)O)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O | 3879.8 | Semi standard non polar | 33892256 |
| Uridine triphosphate,1TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O)O)O[C@H]1N1C=CC(=O)[NH]C1=O | 3871.8 | Semi standard non polar | 33892256 |
| Uridine triphosphate,1TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(=O)[NH]C2=O)[C@H](O)[C@@H]1O)OP(=O)(O)OP(=O)(O)O | 3964.0 | Semi standard non polar | 33892256 |
| Uridine triphosphate,1TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)OP(=O)(OP(=O)(O)O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(=O)[NH]C2=O)[C@H](O)[C@@H]1O | 3973.7 | Semi standard non polar | 33892256 |
| Uridine triphosphate,1TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)OP(=O)(O)OP(=O)(O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(=O)[NH]C2=O)[C@H](O)[C@@H]1O | 3952.6 | Semi standard non polar | 33892256 |
| Uridine triphosphate,1TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)N1C(=O)C=CN([C@@H]2O[C@H](COP(=O)(O)OP(=O)(O)OP(=O)(O)O)[C@@H](O)[C@H]2O)C1=O | 3963.8 | Semi standard non polar | 33892256 |
| Uridine triphosphate,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O)O)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O[Si](C)(C)C(C)(C)C | 3926.8 | Semi standard non polar | 33892256 |
| Uridine triphosphate,2TBDMS,isomer #10 | CC(C)(C)[Si](C)(C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(=O)[NH]C2=O)[C@H](O)[C@@H]1O)OP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O | 4060.6 | Semi standard non polar | 33892256 |
| Uridine triphosphate,2TBDMS,isomer #11 | CC(C)(C)[Si](C)(C)OP(=O)(O)OP(=O)(O)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(=O)[NH]C2=O)[C@H](O)[C@@H]1O)O[Si](C)(C)C(C)(C)C | 4058.1 | Semi standard non polar | 33892256 |
| Uridine triphosphate,2TBDMS,isomer #12 | CC(C)(C)[Si](C)(C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(=O)N([Si](C)(C)C(C)(C)C)C2=O)[C@H](O)[C@@H]1O)OP(=O)(O)OP(=O)(O)O | 4108.9 | Semi standard non polar | 33892256 |
| Uridine triphosphate,2TBDMS,isomer #13 | CC(C)(C)[Si](C)(C)OP(=O)(O)OP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(=O)[NH]C2=O)[C@H](O)[C@@H]1O | 4060.5 | Semi standard non polar | 33892256 |
| Uridine triphosphate,2TBDMS,isomer #14 | CC(C)(C)[Si](C)(C)OP(=O)(OP(=O)(O)O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(=O)N([Si](C)(C)C(C)(C)C)C2=O)[C@H](O)[C@@H]1O | 4114.4 | Semi standard non polar | 33892256 |
| Uridine triphosphate,2TBDMS,isomer #15 | CC(C)(C)[Si](C)(C)OP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(=O)[NH]C2=O)[C@H](O)[C@@H]1O | 4046.8 | Semi standard non polar | 33892256 |
| Uridine triphosphate,2TBDMS,isomer #16 | CC(C)(C)[Si](C)(C)OP(=O)(O)OP(=O)(O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(=O)N([Si](C)(C)C(C)(C)C)C2=O)[C@H](O)[C@@H]1O | 4101.7 | Semi standard non polar | 33892256 |
| Uridine triphosphate,2TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)OP(=O)(O)O)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O | 4004.4 | Semi standard non polar | 33892256 |
| Uridine triphosphate,2TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O | 4009.2 | Semi standard non polar | 33892256 |
| Uridine triphosphate,2TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O)O[Si](C)(C)C(C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O | 4005.6 | Semi standard non polar | 33892256 |
| Uridine triphosphate,2TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O)O)O[C@@H](N2C=CC(=O)N([Si](C)(C)C(C)(C)C)C2=O)[C@@H]1O | 4047.7 | Semi standard non polar | 33892256 |
| Uridine triphosphate,2TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)OP(=O)(O)O)O[C@H]1N1C=CC(=O)[NH]C1=O | 4000.9 | Semi standard non polar | 33892256 |
| Uridine triphosphate,2TBDMS,isomer #7 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O)O[C@H]1N1C=CC(=O)[NH]C1=O | 4007.2 | Semi standard non polar | 33892256 |
| Uridine triphosphate,2TBDMS,isomer #8 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O)O[Si](C)(C)C(C)(C)C)O[C@H]1N1C=CC(=O)[NH]C1=O | 4003.0 | Semi standard non polar | 33892256 |
| Uridine triphosphate,2TBDMS,isomer #9 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O)O)O[C@H]1N1C=CC(=O)N([Si](C)(C)C(C)(C)C)C1=O | 4041.1 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)OP(=O)(O)O)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O[Si](C)(C)C(C)(C)C | 4097.3 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)OP(=O)(O)O)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O[Si](C)(C)C(C)(C)C | 4119.7 | Standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)OP(=O)(O)O)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O[Si](C)(C)C(C)(C)C | 5476.3 | Standard polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #10 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O | 4150.0 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #10 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O | 4104.1 | Standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #10 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O | 5376.2 | Standard polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #11 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O)O[Si](C)(C)C(C)(C)C)O[C@@H](N2C=CC(=O)N([Si](C)(C)C(C)(C)C)C2=O)[C@@H]1O | 4187.6 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #11 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O)O[Si](C)(C)C(C)(C)C)O[C@@H](N2C=CC(=O)N([Si](C)(C)C(C)(C)C)C2=O)[C@@H]1O | 4190.9 | Standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #11 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O)O[Si](C)(C)C(C)(C)C)O[C@@H](N2C=CC(=O)N([Si](C)(C)C(C)(C)C)C2=O)[C@@H]1O | 5565.4 | Standard polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #12 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O)O[C@H]1N1C=CC(=O)[NH]C1=O | 4145.6 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #12 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O)O[C@H]1N1C=CC(=O)[NH]C1=O | 4178.9 | Standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #12 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O)O[C@H]1N1C=CC(=O)[NH]C1=O | 5247.5 | Standard polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #13 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)OP(=O)(O)O[Si](C)(C)C(C)(C)C)O[C@H]1N1C=CC(=O)[NH]C1=O | 4159.5 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #13 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)OP(=O)(O)O[Si](C)(C)C(C)(C)C)O[C@H]1N1C=CC(=O)[NH]C1=O | 4171.4 | Standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #13 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)OP(=O)(O)O[Si](C)(C)C(C)(C)C)O[C@H]1N1C=CC(=O)[NH]C1=O | 5361.0 | Standard polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #14 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)OP(=O)(O)O)O[C@H]1N1C=CC(=O)N([Si](C)(C)C(C)(C)C)C1=O | 4198.9 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #14 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)OP(=O)(O)O)O[C@H]1N1C=CC(=O)N([Si](C)(C)C(C)(C)C)C1=O | 4200.0 | Standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #14 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)OP(=O)(O)O)O[C@H]1N1C=CC(=O)N([Si](C)(C)C(C)(C)C)C1=O | 5546.2 | Standard polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #15 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O[Si](C)(C)C(C)(C)C)O[C@H]1N1C=CC(=O)[NH]C1=O | 4152.6 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #15 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O[Si](C)(C)C(C)(C)C)O[C@H]1N1C=CC(=O)[NH]C1=O | 4181.4 | Standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #15 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O[Si](C)(C)C(C)(C)C)O[C@H]1N1C=CC(=O)[NH]C1=O | 5289.0 | Standard polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #16 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O)O[C@H]1N1C=CC(=O)N([Si](C)(C)C(C)(C)C)C1=O | 4197.7 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #16 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O)O[C@H]1N1C=CC(=O)N([Si](C)(C)C(C)(C)C)C1=O | 4210.2 | Standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #16 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O)O[C@H]1N1C=CC(=O)N([Si](C)(C)C(C)(C)C)C1=O | 5480.6 | Standard polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #17 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)O[C@H]1N1C=CC(=O)[NH]C1=O | 4150.9 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #17 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)O[C@H]1N1C=CC(=O)[NH]C1=O | 4141.2 | Standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #17 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)O[C@H]1N1C=CC(=O)[NH]C1=O | 5393.9 | Standard polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #18 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O)O[Si](C)(C)C(C)(C)C)O[C@H]1N1C=CC(=O)N([Si](C)(C)C(C)(C)C)C1=O | 4192.0 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #18 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O)O[Si](C)(C)C(C)(C)C)O[C@H]1N1C=CC(=O)N([Si](C)(C)C(C)(C)C)C1=O | 4203.1 | Standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #18 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@H](O)[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O)O[Si](C)(C)C(C)(C)C)O[C@H]1N1C=CC(=O)N([Si](C)(C)C(C)(C)C)C1=O | 5582.1 | Standard polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #19 | CC(C)(C)[Si](C)(C)OP(=O)(O)OP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(=O)[NH]C2=O)[C@H](O)[C@@H]1O)O[Si](C)(C)C(C)(C)C | 4185.8 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #19 | CC(C)(C)[Si](C)(C)OP(=O)(O)OP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(=O)[NH]C2=O)[C@H](O)[C@@H]1O)O[Si](C)(C)C(C)(C)C | 4200.5 | Standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #19 | CC(C)(C)[Si](C)(C)OP(=O)(O)OP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(=O)[NH]C2=O)[C@H](O)[C@@H]1O)O[Si](C)(C)C(C)(C)C | 5122.7 | Standard polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O[Si](C)(C)C(C)(C)C | 4103.6 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O[Si](C)(C)C(C)(C)C | 4125.1 | Standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O[Si](C)(C)C(C)(C)C | 5418.9 | Standard polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #20 | CC(C)(C)[Si](C)(C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(=O)N([Si](C)(C)C(C)(C)C)C2=O)[C@H](O)[C@@H]1O)OP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O | 4228.4 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #20 | CC(C)(C)[Si](C)(C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(=O)N([Si](C)(C)C(C)(C)C)C2=O)[C@H](O)[C@@H]1O)OP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O | 4248.6 | Standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #20 | CC(C)(C)[Si](C)(C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(=O)N([Si](C)(C)C(C)(C)C)C2=O)[C@H](O)[C@@H]1O)OP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O | 5323.2 | Standard polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #21 | CC(C)(C)[Si](C)(C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(=O)[NH]C2=O)[C@H](O)[C@@H]1O)OP(=O)(O)OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 4195.0 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #21 | CC(C)(C)[Si](C)(C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(=O)[NH]C2=O)[C@H](O)[C@@H]1O)OP(=O)(O)OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 4154.7 | Standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #21 | CC(C)(C)[Si](C)(C)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(=O)[NH]C2=O)[C@H](O)[C@@H]1O)OP(=O)(O)OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 5210.0 | Standard polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #22 | CC(C)(C)[Si](C)(C)OP(=O)(O)OP(=O)(O)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(=O)N([Si](C)(C)C(C)(C)C)C2=O)[C@H](O)[C@@H]1O)O[Si](C)(C)C(C)(C)C | 4225.4 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #22 | CC(C)(C)[Si](C)(C)OP(=O)(O)OP(=O)(O)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(=O)N([Si](C)(C)C(C)(C)C)C2=O)[C@H](O)[C@@H]1O)O[Si](C)(C)C(C)(C)C | 4232.8 | Standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #22 | CC(C)(C)[Si](C)(C)OP(=O)(O)OP(=O)(O)OP(=O)(OC[C@H]1O[C@@H](N2C=CC(=O)N([Si](C)(C)C(C)(C)C)C2=O)[C@H](O)[C@@H]1O)O[Si](C)(C)C(C)(C)C | 5430.2 | Standard polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #23 | CC(C)(C)[Si](C)(C)OP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(=O)[NH]C2=O)[C@H](O)[C@@H]1O | 4181.9 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #23 | CC(C)(C)[Si](C)(C)OP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(=O)[NH]C2=O)[C@H](O)[C@@H]1O | 4161.6 | Standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #23 | CC(C)(C)[Si](C)(C)OP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(=O)[NH]C2=O)[C@H](O)[C@@H]1O | 5168.2 | Standard polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #24 | CC(C)(C)[Si](C)(C)OP(=O)(O)OP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(=O)N([Si](C)(C)C(C)(C)C)C2=O)[C@H](O)[C@@H]1O | 4228.2 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #24 | CC(C)(C)[Si](C)(C)OP(=O)(O)OP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(=O)N([Si](C)(C)C(C)(C)C)C2=O)[C@H](O)[C@@H]1O | 4250.3 | Standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #24 | CC(C)(C)[Si](C)(C)OP(=O)(O)OP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(=O)N([Si](C)(C)C(C)(C)C)C2=O)[C@H](O)[C@@H]1O | 5366.4 | Standard polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #25 | CC(C)(C)[Si](C)(C)OP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(=O)N([Si](C)(C)C(C)(C)C)C2=O)[C@H](O)[C@@H]1O | 4220.8 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #25 | CC(C)(C)[Si](C)(C)OP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(=O)N([Si](C)(C)C(C)(C)C)C2=O)[C@H](O)[C@@H]1O | 4205.7 | Standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #25 | CC(C)(C)[Si](C)(C)OP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)OP(=O)(O)OC[C@H]1O[C@@H](N2C=CC(=O)N([Si](C)(C)C(C)(C)C)C2=O)[C@H](O)[C@@H]1O | 5462.9 | Standard polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O)O[Si](C)(C)C(C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O[Si](C)(C)C(C)(C)C | 4112.5 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O)O[Si](C)(C)C(C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O[Si](C)(C)C(C)(C)C | 4124.6 | Standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O)O[Si](C)(C)C(C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O[Si](C)(C)C(C)(C)C | 5523.5 | Standard polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O)O)O[C@@H](N2C=CC(=O)N([Si](C)(C)C(C)(C)C)C2=O)[C@@H]1O[Si](C)(C)C(C)(C)C | 4150.2 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O)O)O[C@@H](N2C=CC(=O)N([Si](C)(C)C(C)(C)C)C2=O)[C@@H]1O[Si](C)(C)C(C)(C)C | 4153.3 | Standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O)OP(=O)(O)O)O[C@@H](N2C=CC(=O)N([Si](C)(C)C(C)(C)C)C2=O)[C@@H]1O[Si](C)(C)C(C)(C)C | 5701.7 | Standard polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O | 4144.4 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O | 4144.3 | Standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O | 5219.9 | Standard polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)OP(=O)(O)O[Si](C)(C)C(C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O | 4160.2 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)OP(=O)(O)O[Si](C)(C)C(C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O | 4134.5 | Standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)OP(=O)(O)O[Si](C)(C)C(C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O | 5333.1 | Standard polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #7 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)OP(=O)(O)O)O[C@@H](N2C=CC(=O)N([Si](C)(C)C(C)(C)C)C2=O)[C@@H]1O | 4193.9 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #7 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)OP(=O)(O)O)O[C@@H](N2C=CC(=O)N([Si](C)(C)C(C)(C)C)C2=O)[C@@H]1O | 4187.9 | Standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #7 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)OP(=O)(O)O)O[C@@H](N2C=CC(=O)N([Si](C)(C)C(C)(C)C)C2=O)[C@@H]1O | 5519.0 | Standard polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #8 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O[Si](C)(C)C(C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O | 4151.7 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #8 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O[Si](C)(C)C(C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O | 4145.2 | Standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #8 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O[Si](C)(C)C(C)(C)C)O[C@@H](N2C=CC(=O)[NH]C2=O)[C@@H]1O | 5272.6 | Standard polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #9 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O)O[C@@H](N2C=CC(=O)N([Si](C)(C)C(C)(C)C)C2=O)[C@@H]1O | 4192.3 | Semi standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #9 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O)O[C@@H](N2C=CC(=O)N([Si](C)(C)C(C)(C)C)C2=O)[C@@H]1O | 4199.1 | Standard non polar | 33892256 |
| Uridine triphosphate,3TBDMS,isomer #9 | CC(C)(C)[Si](C)(C)O[C@@H]1[C@@H](COP(=O)(O)OP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O)O[C@@H](N2C=CC(=O)N([Si](C)(C)C(C)(C)C)C2=O)[C@@H]1O | 5465.2 | Standard polar | 33892256 |