| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected and Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2023-02-21 17:15:06 UTC |
|---|
| HMDB ID | HMDB0000719 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | L-Homoserine |
|---|
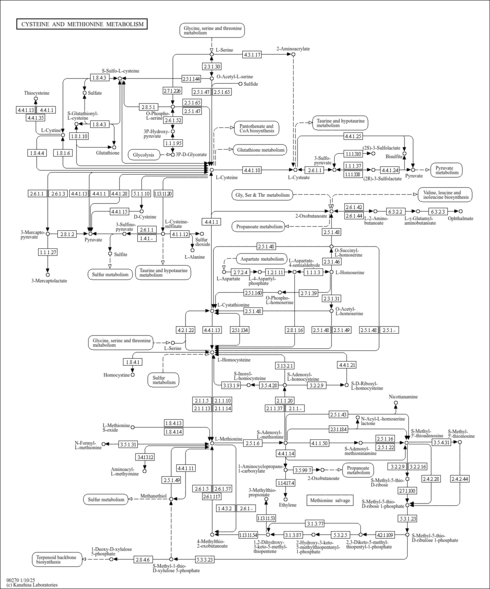
| Description | Homoserine is a more reactive variant of the amino acid serine. In this variant, the hydroxyl side chain contains an additional CH2 group which brings the hydroxyl group closer to its own carboxyl group, allowing it to chemically react to form a five-membered ring. This occurs at the point that amino acids normally join to their neighbours in a peptide bond. Homoserine is therefore unsuitable for forming proteins and has been eliminated from the repertoire of amino acids used by living things. Homoserine is the final product on the C-terminal end of the N-terminal fragment following a cyanogen bromide cleavage. (wikipedia). Homoserine is also a microbial metabolite. |
|---|
| Structure | InChI=1S/C4H9NO3/c5-3(1-2-6)4(7)8/h3,6H,1-2,5H2,(H,7,8)/t3-/m0/s1 |
|---|
| Synonyms | | Value | Source |
|---|
| (2S)-2-Amino-4-hydroxybutanoic acid | ChEBI | | 2-Amino-4-hydroxybutanoic acid | ChEBI | | 2-Amino-4-hydroxybutyric acid | ChEBI | | Homoserine | ChEBI | | (2S)-2-Amino-4-hydroxybutanoate | Generator | | 2-Amino-4-hydroxybutanoate | Generator | | 2-Amino-4-hydroxybutyrate | Generator | | (S)-2-Amino-4-hydroxy-butanoate | HMDB | | (S)-2-Amino-4-hydroxy-butanoic acid | HMDB | | (S)-2-Amino-4-hydroxybutanoate | HMDB | | (S)-2-Amino-4-hydroxybutanoic acid | HMDB | | (S)-Homoserine | HMDB | | 2-Amino-4-hydroxy-butyrate | HMDB | | 2-Amino-4-hydroxy-butyric acid | HMDB | | 2-Amino-4-hydroxy-L-butyrate | HMDB | | 2-Amino-4-hydroxy-L-butyric acid | HMDB | | Homoserine L-isomer | HMDB | | L Isomer OF homoserine | HMDB | | L-Isomer OF homoserine | HMDB |
|
|---|
| Chemical Formula | C4H9NO3 |
|---|
| Average Molecular Weight | 119.1192 |
|---|
| Monoisotopic Molecular Weight | 119.058243159 |
|---|
| IUPAC Name | (2S)-2-amino-4-hydroxybutanoic acid |
|---|
| Traditional Name | L-homoserine |
|---|
| CAS Registry Number | 672-15-1 |
|---|
| SMILES | N[C@@H](CCO)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C4H9NO3/c5-3(1-2-6)4(7)8/h3,6H,1-2,5H2,(H,7,8)/t3-/m0/s1 |
|---|
| InChI Key | UKAUYVFTDYCKQA-VKHMYHEASA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as l-alpha-amino acids. These are alpha amino acids which have the L-configuration of the alpha-carbon atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | L-alpha-amino acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - L-alpha-amino acid
- Short-chain hydroxy acid
- Fatty acid
- 1,3-aminoalcohol
- Amino acid
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Organic nitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Primary amine
- Primary alcohol
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Primary aliphatic amine
- Organic oxygen compound
- Carbonyl group
- Amine
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | Not Available |
|---|
| Process | Not Available |
|---|
| Role | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | |
|---|
| Experimental Chromatographic Properties | Experimental Collision Cross Sections |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Retention Times Underivatized| Chromatographic Method | Retention Time | Reference |
|---|
| Measured using a Waters Acquity ultraperformance liquid chromatography (UPLC) ethylene-bridged hybrid (BEH) C18 column (100 mm × 2.1 mm; 1.7 μmparticle diameter). Predicted by Afia on May 17, 2022. Predicted by Afia on May 17, 2022. | 0.64 minutes | 32390414 | | Predicted by Siyang on May 30, 2022 | 8.9114 minutes | 33406817 | | Predicted by Siyang using ReTip algorithm on June 8, 2022 | 8.17 minutes | 32390414 | | AjsUoB = Accucore 150 Amide HILIC with 10mM Ammonium Formate, 0.1% Formic Acid | 407.9 seconds | 40023050 | | Fem_Long = Waters ACQUITY UPLC HSS T3 C18 with Water:MeOH and 0.1% Formic Acid | 441.1 seconds | 40023050 | | Fem_Lipids = Ascentis Express C18 with (60:40 water:ACN):(90:10 IPA:ACN) and 10mM NH4COOH + 0.1% Formic Acid | 326.5 seconds | 40023050 | | Life_Old = Waters ACQUITY UPLC BEH C18 with Water:(20:80 acetone:ACN) and 0.1% Formic Acid | 40.3 seconds | 40023050 | | Life_New = RP Waters ACQUITY UPLC HSS T3 C18 with Water:(30:70 MeOH:ACN) and 0.1% Formic Acid | 210.0 seconds | 40023050 | | RIKEN = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 78.4 seconds | 40023050 | | Eawag_XBridgeC18 = XBridge C18 3.5u 2.1x50 mm with Water:MeOH and 0.1% Formic Acid | 284.6 seconds | 40023050 | | BfG_NTS_RP1 =Agilent Zorbax Eclipse Plus C18 (2.1 mm x 150 mm, 3.5 um) with Water:ACN and 0.1% Formic Acid | 219.9 seconds | 40023050 | | HILIC_BDD_2 = Merck SeQuant ZIC-HILIC with ACN(0.1% formic acid):water(16 mM ammonium formate) | 883.5 seconds | 40023050 | | UniToyama_Atlantis = RP Waters Atlantis T3 (2.1 x 150 mm, 5 um) with ACN:Water and 0.1% Formic Acid | 566.3 seconds | 40023050 | | BDD_C18 = Hypersil Gold 1.9µm C18 with Water:ACN and 0.1% Formic Acid | 38.6 seconds | 40023050 | | UFZ_Phenomenex = Kinetex Core-Shell C18 2.6 um, 3.0 x 100 mm, Phenomenex with Water:MeOH and 0.1% Formic Acid | 657.9 seconds | 40023050 | | SNU_RIKEN_POS = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 209.2 seconds | 40023050 | | RPMMFDA = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 308.6 seconds | 40023050 | | MTBLS87 = Merck SeQuant ZIC-pHILIC column with ACN:Water and :ammonium carbonate | 745.8 seconds | 40023050 | | KI_GIAR_zic_HILIC_pH2_7 = Merck SeQuant ZIC-HILIC with ACN:Water and 0.1% FA | 546.9 seconds | 40023050 | | Meister zic-pHILIC pH9.3 = Merck SeQuant ZIC-pHILIC column with ACN:Water 5mM NH4Ac pH9.3 and 5mM ammonium acetate in water | 404.2 seconds | 40023050 |
Predicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| L-Homoserine,1TMS,isomer #1 | C[Si](C)(C)OCC[C@H](N)C(=O)O | 1313.9 | Semi standard non polar | 33892256 | | L-Homoserine,1TMS,isomer #2 | C[Si](C)(C)OC(=O)[C@@H](N)CCO | 1231.9 | Semi standard non polar | 33892256 | | L-Homoserine,1TMS,isomer #3 | C[Si](C)(C)N[C@@H](CCO)C(=O)O | 1350.8 | Semi standard non polar | 33892256 | | L-Homoserine,2TMS,isomer #1 | C[Si](C)(C)OCC[C@H](N)C(=O)O[Si](C)(C)C | 1354.9 | Semi standard non polar | 33892256 | | L-Homoserine,2TMS,isomer #2 | C[Si](C)(C)N[C@@H](CCO[Si](C)(C)C)C(=O)O | 1426.7 | Semi standard non polar | 33892256 | | L-Homoserine,2TMS,isomer #3 | C[Si](C)(C)N[C@@H](CCO)C(=O)O[Si](C)(C)C | 1383.3 | Semi standard non polar | 33892256 | | L-Homoserine,2TMS,isomer #4 | C[Si](C)(C)N([C@@H](CCO)C(=O)O)[Si](C)(C)C | 1527.7 | Semi standard non polar | 33892256 | | L-Homoserine,3TMS,isomer #1 | C[Si](C)(C)N[C@@H](CCO[Si](C)(C)C)C(=O)O[Si](C)(C)C | 1471.3 | Semi standard non polar | 33892256 | | L-Homoserine,3TMS,isomer #1 | C[Si](C)(C)N[C@@H](CCO[Si](C)(C)C)C(=O)O[Si](C)(C)C | 1519.5 | Standard non polar | 33892256 | | L-Homoserine,3TMS,isomer #1 | C[Si](C)(C)N[C@@H](CCO[Si](C)(C)C)C(=O)O[Si](C)(C)C | 1571.5 | Standard polar | 33892256 | | L-Homoserine,3TMS,isomer #2 | C[Si](C)(C)OCC[C@@H](C(=O)O)N([Si](C)(C)C)[Si](C)(C)C | 1609.5 | Semi standard non polar | 33892256 | | L-Homoserine,3TMS,isomer #2 | C[Si](C)(C)OCC[C@@H](C(=O)O)N([Si](C)(C)C)[Si](C)(C)C | 1561.4 | Standard non polar | 33892256 | | L-Homoserine,3TMS,isomer #2 | C[Si](C)(C)OCC[C@@H](C(=O)O)N([Si](C)(C)C)[Si](C)(C)C | 1710.8 | Standard polar | 33892256 | | L-Homoserine,3TMS,isomer #3 | C[Si](C)(C)OC(=O)[C@H](CCO)N([Si](C)(C)C)[Si](C)(C)C | 1556.8 | Semi standard non polar | 33892256 | | L-Homoserine,3TMS,isomer #3 | C[Si](C)(C)OC(=O)[C@H](CCO)N([Si](C)(C)C)[Si](C)(C)C | 1509.5 | Standard non polar | 33892256 | | L-Homoserine,3TMS,isomer #3 | C[Si](C)(C)OC(=O)[C@H](CCO)N([Si](C)(C)C)[Si](C)(C)C | 1650.2 | Standard polar | 33892256 | | L-Homoserine,4TMS,isomer #1 | C[Si](C)(C)OCC[C@@H](C(=O)O[Si](C)(C)C)N([Si](C)(C)C)[Si](C)(C)C | 1660.7 | Semi standard non polar | 33892256 | | L-Homoserine,4TMS,isomer #1 | C[Si](C)(C)OCC[C@@H](C(=O)O[Si](C)(C)C)N([Si](C)(C)C)[Si](C)(C)C | 1634.1 | Standard non polar | 33892256 | | L-Homoserine,4TMS,isomer #1 | C[Si](C)(C)OCC[C@@H](C(=O)O[Si](C)(C)C)N([Si](C)(C)C)[Si](C)(C)C | 1536.9 | Standard polar | 33892256 | | L-Homoserine,1TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OCC[C@H](N)C(=O)O | 1555.2 | Semi standard non polar | 33892256 | | L-Homoserine,1TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OC(=O)[C@@H](N)CCO | 1469.3 | Semi standard non polar | 33892256 | | L-Homoserine,1TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)N[C@@H](CCO)C(=O)O | 1605.6 | Semi standard non polar | 33892256 | | L-Homoserine,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OCC[C@H](N)C(=O)O[Si](C)(C)C(C)(C)C | 1801.9 | Semi standard non polar | 33892256 | | L-Homoserine,2TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)N[C@@H](CCO[Si](C)(C)C(C)(C)C)C(=O)O | 1871.4 | Semi standard non polar | 33892256 | | L-Homoserine,2TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)N[C@@H](CCO)C(=O)O[Si](C)(C)C(C)(C)C | 1810.4 | Semi standard non polar | 33892256 | | L-Homoserine,2TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)N([C@@H](CCO)C(=O)O)[Si](C)(C)C(C)(C)C | 1989.4 | Semi standard non polar | 33892256 | | L-Homoserine,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)N[C@@H](CCO[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C | 2092.9 | Semi standard non polar | 33892256 | | L-Homoserine,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)N[C@@H](CCO[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C | 2107.5 | Standard non polar | 33892256 | | L-Homoserine,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)N[C@@H](CCO[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C | 1979.7 | Standard polar | 33892256 | | L-Homoserine,3TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OCC[C@@H](C(=O)O)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2247.4 | Semi standard non polar | 33892256 | | L-Homoserine,3TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OCC[C@@H](C(=O)O)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2166.2 | Standard non polar | 33892256 | | L-Homoserine,3TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OCC[C@@H](C(=O)O)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2041.0 | Standard polar | 33892256 | | L-Homoserine,3TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC(=O)[C@H](CCO)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2219.6 | Semi standard non polar | 33892256 | | L-Homoserine,3TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC(=O)[C@H](CCO)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2130.0 | Standard non polar | 33892256 | | L-Homoserine,3TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC(=O)[C@H](CCO)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 1998.5 | Standard polar | 33892256 | | L-Homoserine,4TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OCC[C@@H](C(=O)O[Si](C)(C)C(C)(C)C)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2508.1 | Semi standard non polar | 33892256 | | L-Homoserine,4TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OCC[C@@H](C(=O)O[Si](C)(C)C(C)(C)C)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2387.5 | Standard non polar | 33892256 | | L-Homoserine,4TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OCC[C@@H](C(=O)O[Si](C)(C)C(C)(C)C)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2050.8 | Standard polar | 33892256 |
|
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental GC-MS | GC-MS Spectrum - L-Homoserine GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (3 TMS) | splash10-0v00-0930000000-3346aa2189b174721b86 | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - L-Homoserine GC-MS (2 TMS) | splash10-0udj-1900000000-88ffce416068e87d5c9e | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - L-Homoserine GC-MS (3 TMS) | splash10-0gdi-1960000000-b676faeb6cb80ca15570 | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - L-Homoserine GC-EI-TOF (Non-derivatized) | splash10-0v00-0930000000-3346aa2189b174721b86 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - L-Homoserine GC-MS (Non-derivatized) | splash10-0udj-1900000000-88ffce416068e87d5c9e | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - L-Homoserine GC-MS (Non-derivatized) | splash10-0gdi-1960000000-b676faeb6cb80ca15570 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - L-Homoserine GC-EI-TOF (Non-derivatized) | splash10-0gdi-0930000000-6216c27e0d6581b937aa | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - L-Homoserine GC-EI-TOF (Non-derivatized) | splash10-0udj-0900000000-d59a1b29c14ce935a8a2 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - L-Homoserine GC-EI-TOF (Non-derivatized) | splash10-0ufv-0930000000-6efd3a5ade5c7320afb0 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - L-Homoserine GC-MS (Non-derivatized) - 70eV, Positive | splash10-00dl-9100000000-d741144429bbc0c89fb2 | 2016-09-22 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - L-Homoserine GC-MS (2 TMS) - 70eV, Positive | splash10-0fk9-9530000000-9f3f4a1b429b17df7168 | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - L-Homoserine GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - L-Homoserine GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - L-Homoserine GC-MS (TMS_1_1) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - L-Homoserine GC-MS (TMS_1_2) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - L-Homoserine GC-MS (TMS_1_3) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - L-Homoserine GC-MS (TMS_2_2) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - L-Homoserine GC-MS (TMS_2_3) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - L-Homoserine GC-MS (TMS_2_4) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - L-Homoserine GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - L-Homoserine GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - L-Homoserine GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - L-Homoserine GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - L-Homoserine GC-MS (TBDMS_2_2) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - L-Homoserine GC-MS (TBDMS_2_3) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental LC-MS/MS | LC-MS/MS Spectrum - L-Homoserine Quattro_QQQ 10V, Positive-QTOF (Annotated) | splash10-00di-9200000000-89f42491f91db334ecfc | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - L-Homoserine Quattro_QQQ 25V, Positive-QTOF (Annotated) | splash10-0a4i-9000000000-d1859b3d67ed95698fa3 | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - L-Homoserine Quattro_QQQ 40V, Positive-QTOF (Annotated) | splash10-0abc-9100000000-eb77443ca02c8ccbe33a | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - L-Homoserine LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive-QTOF | splash10-00di-0900000000-395a3c4889481d35a3f7 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - L-Homoserine LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive-QTOF | splash10-00di-9300000000-634c194ce44ff0b3755f | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - L-Homoserine LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive-QTOF | splash10-004i-9300000000-0c6e3c4dd011ef8c64fd | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - L-Homoserine LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive-QTOF | splash10-000i-9000000000-d6e4f803999f61b58a6e | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - L-Homoserine LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive-QTOF | splash10-00di-0900000000-45ed6aea7015ba6e6ca5 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - L-Homoserine LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive-QTOF | splash10-00di-9400000000-30b57c9aabcc05806ce4 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - L-Homoserine LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive-QTOF | splash10-0fk9-9400000000-74b23d9b0819343ff386 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - L-Homoserine LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive-QTOF | splash10-000i-9000000000-cde89cc67f5c874ed39c | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - L-Homoserine LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative-QTOF | splash10-02t9-0891200000-72078b7028ff67e8adac | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - L-Homoserine LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative-QTOF | splash10-0002-9000000000-178d6a086fe4d5123d31 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - L-Homoserine LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative-QTOF | splash10-00kf-0900000000-1281df962d8640156255 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - L-Homoserine LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative-QTOF | splash10-02t9-0390000000-0afae0c03453d26542e6 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - L-Homoserine LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative-QTOF | splash10-014i-0891100000-48060b947316dc631321 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - L-Homoserine LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative-QTOF | splash10-0002-9000000000-3427ff1b0434696ae956 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - L-Homoserine LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative-QTOF | splash10-014i-0900000000-fd0f3bbc1a17c20a2475 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - L-Homoserine LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative-QTOF | splash10-03xr-0390000000-5a4fde017ce3426e8cd5 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - L-Homoserine LC-ESI-QQ (API3000, Applied Biosystems) 10V, Positive-QTOF | splash10-00di-5900000000-a5903a71e92a78624d03 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - L-Homoserine LC-ESI-QQ (API3000, Applied Biosystems) 20V, Positive-QTOF | splash10-00di-9100000000-422851033dffe854f591 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - L-Homoserine LC-ESI-QQ (API3000, Applied Biosystems) 30V, Positive-QTOF | splash10-0ab9-9000000000-cd7b70e1ead33d38a19f | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - L-Homoserine LC-ESI-QQ (API3000, Applied Biosystems) 40V, Positive-QTOF | splash10-0a4l-9000000000-481d61466b1d0af71cc2 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - L-Homoserine LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Positive-QTOF | splash10-00di-9200000000-afddddfff24e50b7f835 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - L-Homoserine LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Negative-QTOF | splash10-0gb9-1900000000-89eb5226702ae527a2be | 2012-08-31 | HMDB team, MONA | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Experimental 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, experimental) | 2012-12-04 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Experimental 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | 2012-12-05 | Wishart Lab | View Spectrum |
IR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M-H]-) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+H]+) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+Na]+) | 2023-02-03 | FELIX lab | View Spectrum |
|
|---|
| Disease References | | Colorectal cancer |
|---|
- Sinha R, Ahn J, Sampson JN, Shi J, Yu G, Xiong X, Hayes RB, Goedert JJ: Fecal Microbiota, Fecal Metabolome, and Colorectal Cancer Interrelations. PLoS One. 2016 Mar 25;11(3):e0152126. doi: 10.1371/journal.pone.0152126. eCollection 2016. [PubMed:27015276 ]
- Brown DG, Rao S, Weir TL, O'Malia J, Bazan M, Brown RJ, Ryan EP: Metabolomics and metabolic pathway networks from human colorectal cancers, adjacent mucosa, and stool. Cancer Metab. 2016 Jun 6;4:11. doi: 10.1186/s40170-016-0151-y. eCollection 2016. [PubMed:27275383 ]
- Goedert JJ, Sampson JN, Moore SC, Xiao Q, Xiong X, Hayes RB, Ahn J, Shi J, Sinha R: Fecal metabolomics: assay performance and association with colorectal cancer. Carcinogenesis. 2014 Sep;35(9):2089-96. doi: 10.1093/carcin/bgu131. Epub 2014 Jul 18. [PubMed:25037050 ]
| | Crohn's disease |
|---|
- Kolho KL, Pessia A, Jaakkola T, de Vos WM, Velagapudi V: Faecal and Serum Metabolomics in Paediatric Inflammatory Bowel Disease. J Crohns Colitis. 2017 Mar 1;11(3):321-334. doi: 10.1093/ecco-jcc/jjw158. [PubMed:27609529 ]
| | Ulcerative colitis |
|---|
- Kolho KL, Pessia A, Jaakkola T, de Vos WM, Velagapudi V: Faecal and Serum Metabolomics in Paediatric Inflammatory Bowel Disease. J Crohns Colitis. 2017 Mar 1;11(3):321-334. doi: 10.1093/ecco-jcc/jjw158. [PubMed:27609529 ]
| | Eosinophilic esophagitis |
|---|
- Slae, M., Huynh, H., Wishart, D.S. (2014). Analysis of 30 normal pediatric urine samples via NMR spectroscopy (unpublished work). NA.
|
|
|---|
| General References | - Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM: Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009 Feb 12;457(7231):910-4. doi: 10.1038/nature07762. [PubMed:19212411 ]
- Shoemaker JD, Elliott WH: Automated screening of urine samples for carbohydrates, organic and amino acids after treatment with urease. J Chromatogr. 1991 Jan 2;562(1-2):125-38. [PubMed:2026685 ]
- Gazarian KG, Gening LV, Gazarian TG: L-Homoserine: a novel excreted metabolic marker of hepatitis B abnormally produced in liver from methionine. Med Hypotheses. 2002 Apr;58(4):279-83. [PubMed:12027520 ]
- Compagnini A, Cunsolo V, Foti S, Saletti R: Improved accuracy in the matrix-assisted laser desorption/ionization-mass spectrometry determination of the molecular mass of cyanogen bromide fragments of proteins by post-cleavage reaction with tris(hydroxymethyl)aminomethane. Proteomics. 2001 Aug;1(8):967-74. [PubMed:11683513 ]
|
|---|