| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected and Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2023-02-21 17:14:27 UTC |
|---|
| HMDB ID | HMDB0000002 |
|---|
| Secondary Accession Numbers | - HMDB00002
- HMDB0060172
- HMDB60172
|
|---|
| Metabolite Identification |
|---|
| Common Name | 1,3-Diaminopropane |
|---|
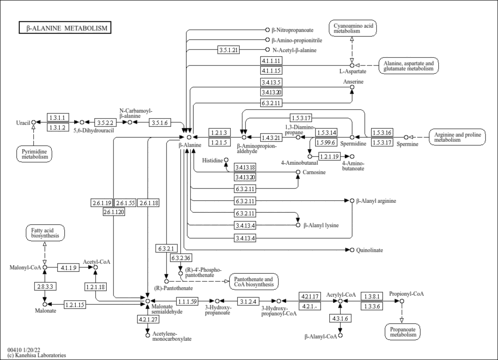
| Description | 1,3-Diaminopropane, also known as DAP or trimethylenediamine, belongs to the class of organic compounds known as monoalkylamines. These are organic compounds containing a primary aliphatic amine group. 1,3-Diaminopropane is a stable, flammable, and highly hygroscopic fluid. It is a polyamine that is normally quite toxic if swallowed, inhaled, or absorbed through the skin. It is a catabolic byproduct of spermidine. It is also a precursor in the enzymatic synthesis of beta-alanine. 1,3-Diaminopropane is involved in the arginine/proline metabolic pathways and the beta-alanine metabolic pathway. 1,3-Diaminopropane has been detected, but not quantified in, several different foods, such as cassava, shiitakes, oyster mushrooms, muscadine grapes, and cinnamons. This could make 1,3-diaminopropane a potential biomarker for the consumption of these foods. |
|---|
| Structure | InChI=1S/C3H10N2/c4-2-1-3-5/h1-5H2 |
|---|
| Synonyms | | Value | Source |
|---|
| 1,3-Propanediamine | ChEBI | | 1,3-Propylenediamine | ChEBI | | Propane-1,3-diamine | ChEBI | | tn | ChEBI | | Trimethylenediamine | Kegg | | 1,3-Diaminepropane | MeSH | | Trimethylenediamine dihydrochloride | MeSH | | Trimethylenediamine hydrochloride | MeSH | | 1,3-diamino-N-Propane | HMDB | | 1,3-Trimethylenediamine | HMDB | | 3-Aminopropylamine | HMDB | | a,W-Propanediamine | HMDB | | 1,3-Diaminopropane | ChEBI |
|
|---|
| Chemical Formula | C3H10N2 |
|---|
| Average Molecular Weight | 74.1249 |
|---|
| Monoisotopic Molecular Weight | 74.08439833 |
|---|
| IUPAC Name | propane-1,3-diamine |
|---|
| Traditional Name | α,ω-propanediamine |
|---|
| CAS Registry Number | 109-76-2 |
|---|
| SMILES | NCCCN |
|---|
| InChI Identifier | InChI=1S/C3H10N2/c4-2-1-3-5/h1-5H2 |
|---|
| InChI Key | XFNJVJPLKCPIBV-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as monoalkylamines. These are organic compounds containing an primary aliphatic amine group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic nitrogen compounds |
|---|
| Class | Organonitrogen compounds |
|---|
| Sub Class | Amines |
|---|
| Direct Parent | Monoalkylamines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Organopnictogen compound
- Hydrocarbon derivative
- Primary aliphatic amine
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | |
|---|
| Physical Properties |
|---|
| State | Liquid |
|---|
| Experimental Molecular Properties | |
|---|
| Experimental Chromatographic Properties | Not Available |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Retention Times Underivatized| Chromatographic Method | Retention Time | Reference |
|---|
| Measured using a Waters Acquity ultraperformance liquid chromatography (UPLC) ethylene-bridged hybrid (BEH) C18 column (100 mm × 2.1 mm; 1.7 μmparticle diameter). Predicted by Afia on May 17, 2022. | 0.99 minutes | 32390414 | | Predicted by Siyang on May 30, 2022 | 7.3984 minutes | 33406817 | | Predicted by Siyang using ReTip algorithm on June 8, 2022 | 7.72 minutes | 32390414 |
Predicted Kovats Retention IndicesUnderivatized| Metabolite | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| 1,3-Diaminopropane | NCCCN | 1317.2 | Standard polar | 33892256 | | 1,3-Diaminopropane | NCCCN | 736.5 | Standard non polar | 33892256 | | 1,3-Diaminopropane | NCCCN | 732.5 | Semi standard non polar | 33892256 |
Derivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| 1,3-Diaminopropane,1TMS,isomer #1 | C[Si](C)(C)NCCCN | 1032.5 | Semi standard non polar | 33892256 | | 1,3-Diaminopropane,1TMS,isomer #1 | C[Si](C)(C)NCCCN | 1054.4 | Standard non polar | 33892256 | | 1,3-Diaminopropane,1TMS,isomer #1 | C[Si](C)(C)NCCCN | 1668.7 | Standard polar | 33892256 | | 1,3-Diaminopropane,2TMS,isomer #1 | C[Si](C)(C)NCCCN[Si](C)(C)C | 1175.5 | Semi standard non polar | 33892256 | | 1,3-Diaminopropane,2TMS,isomer #1 | C[Si](C)(C)NCCCN[Si](C)(C)C | 1291.5 | Standard non polar | 33892256 | | 1,3-Diaminopropane,2TMS,isomer #1 | C[Si](C)(C)NCCCN[Si](C)(C)C | 1304.0 | Standard polar | 33892256 | | 1,3-Diaminopropane,2TMS,isomer #2 | C[Si](C)(C)N(CCCN)[Si](C)(C)C | 1262.2 | Semi standard non polar | 33892256 | | 1,3-Diaminopropane,2TMS,isomer #2 | C[Si](C)(C)N(CCCN)[Si](C)(C)C | 1290.9 | Standard non polar | 33892256 | | 1,3-Diaminopropane,2TMS,isomer #2 | C[Si](C)(C)N(CCCN)[Si](C)(C)C | 1619.6 | Standard polar | 33892256 | | 1,3-Diaminopropane,3TMS,isomer #1 | C[Si](C)(C)NCCCN([Si](C)(C)C)[Si](C)(C)C | 1415.6 | Semi standard non polar | 33892256 | | 1,3-Diaminopropane,3TMS,isomer #1 | C[Si](C)(C)NCCCN([Si](C)(C)C)[Si](C)(C)C | 1492.1 | Standard non polar | 33892256 | | 1,3-Diaminopropane,3TMS,isomer #1 | C[Si](C)(C)NCCCN([Si](C)(C)C)[Si](C)(C)C | 1347.4 | Standard polar | 33892256 | | 1,3-Diaminopropane,4TMS,isomer #1 | C[Si](C)(C)N(CCCN([Si](C)(C)C)[Si](C)(C)C)[Si](C)(C)C | 1666.7 | Semi standard non polar | 33892256 | | 1,3-Diaminopropane,4TMS,isomer #1 | C[Si](C)(C)N(CCCN([Si](C)(C)C)[Si](C)(C)C)[Si](C)(C)C | 1673.8 | Standard non polar | 33892256 | | 1,3-Diaminopropane,4TMS,isomer #1 | C[Si](C)(C)N(CCCN([Si](C)(C)C)[Si](C)(C)C)[Si](C)(C)C | 1378.4 | Standard polar | 33892256 | | 1,3-Diaminopropane,1TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)NCCCN | 1244.3 | Semi standard non polar | 33892256 | | 1,3-Diaminopropane,1TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)NCCCN | 1280.2 | Standard non polar | 33892256 | | 1,3-Diaminopropane,1TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)NCCCN | 1774.3 | Standard polar | 33892256 | | 1,3-Diaminopropane,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)NCCCN[Si](C)(C)C(C)(C)C | 1659.8 | Semi standard non polar | 33892256 | | 1,3-Diaminopropane,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)NCCCN[Si](C)(C)C(C)(C)C | 1637.0 | Standard non polar | 33892256 | | 1,3-Diaminopropane,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)NCCCN[Si](C)(C)C(C)(C)C | 1562.7 | Standard polar | 33892256 | | 1,3-Diaminopropane,2TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)N(CCCN)[Si](C)(C)C(C)(C)C | 1659.2 | Semi standard non polar | 33892256 | | 1,3-Diaminopropane,2TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)N(CCCN)[Si](C)(C)C(C)(C)C | 1670.0 | Standard non polar | 33892256 | | 1,3-Diaminopropane,2TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)N(CCCN)[Si](C)(C)C(C)(C)C | 1689.3 | Standard polar | 33892256 | | 1,3-Diaminopropane,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)NCCCN([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2074.1 | Semi standard non polar | 33892256 | | 1,3-Diaminopropane,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)NCCCN([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2020.3 | Standard non polar | 33892256 | | 1,3-Diaminopropane,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)NCCCN([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 1724.9 | Standard polar | 33892256 | | 1,3-Diaminopropane,4TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)N(CCCN([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2442.2 | Semi standard non polar | 33892256 | | 1,3-Diaminopropane,4TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)N(CCCN([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2393.9 | Standard non polar | 33892256 | | 1,3-Diaminopropane,4TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)N(CCCN([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 1845.7 | Standard polar | 33892256 |
|
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental GC-MS | GC-MS Spectrum - 1,3-Diaminopropane GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (4 TMS) | splash10-00dr-1900000000-5d8fbabd14e52e72c81e | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - 1,3-Diaminopropane GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (4 TMS) | splash10-00di-1910000000-a06f00628b2c5d1b00d9 | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - 1,3-Diaminopropane GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (Non-derivatized) | splash10-00di-1900000000-380b075ca78246d55dc0 | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - 1,3-Diaminopropane GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (4 TMS) | splash10-00di-6910000000-aff47ff9c6e0a888ca86 | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - 1,3-Diaminopropane GC-MS (4 TMS) | splash10-00di-2910000000-48a829abd35cdd63a4f8 | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - 1,3-Diaminopropane EI-B (Non-derivatized) | splash10-053r-9000000000-0a9fffbfecda98d5cee8 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - 1,3-Diaminopropane EI-B (Non-derivatized) | splash10-053r-9000000000-95ecfa758d5699a04f9f | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - 1,3-Diaminopropane CI-B (Non-derivatized) | splash10-004i-9000000000-aeecaf348c083323da27 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - 1,3-Diaminopropane GC-EI-TOF (Non-derivatized) | splash10-00dr-1900000000-5d8fbabd14e52e72c81e | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - 1,3-Diaminopropane GC-EI-TOF (Non-derivatized) | splash10-00di-1910000000-a06f00628b2c5d1b00d9 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - 1,3-Diaminopropane GC-EI-TOF (Non-derivatized) | splash10-00di-1900000000-380b075ca78246d55dc0 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - 1,3-Diaminopropane GC-EI-TOF (Non-derivatized) | splash10-00di-6910000000-aff47ff9c6e0a888ca86 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - 1,3-Diaminopropane GC-MS (Non-derivatized) | splash10-00di-2910000000-48a829abd35cdd63a4f8 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - 1,3-Diaminopropane GC-EI-TOF (Non-derivatized) | splash10-00dr-2900000000-03807bd96e6c6edb1948 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - 1,3-Diaminopropane GC-EI-TOF (Non-derivatized) | splash10-00dr-2900000000-4d07c816c8f74c70156f | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 1,3-Diaminopropane GC-MS (Non-derivatized) - 70eV, Positive | splash10-001i-9000000000-457e65b34947bb32a5b9 | 2016-09-22 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 1,3-Diaminopropane GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 1,3-Diaminopropane GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-0a59-9000000000-8fe9c1c9a2f7a72fd871 | 2015-03-01 | Not Available | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental LC-MS/MS | LC-MS/MS Spectrum - 1,3-Diaminopropane Quattro_QQQ 10V, Positive-QTOF (Annotated) | splash10-0a4i-9000000000-8df8541d3f928c3f42de | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 1,3-Diaminopropane Quattro_QQQ 25V, Positive-QTOF (Annotated) | splash10-053u-9000000000-7bb459bade8978e3cba4 | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 1,3-Diaminopropane Quattro_QQQ 40V, Positive-QTOF (Annotated) | splash10-001i-9000000000-f6bc23624268bb35a760 | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 1,3-Diaminopropane EI-B (HITACHI RMU-6L) , Positive-QTOF | splash10-053r-9000000000-0a9fffbfecda98d5cee8 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 1,3-Diaminopropane EI-B (HITACHI RMU-6M) , Positive-QTOF | splash10-053r-9000000000-8fdaa5d68825655f464c | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 1,3-Diaminopropane CI-B (HITACHI M-80) , Positive-QTOF | splash10-004i-9000000000-aeecaf348c083323da27 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 1,3-Diaminopropane LC-ESI-QQ (API3000, Applied Biosystems) 10V, Positive-QTOF | splash10-004i-9000000000-1d904fa654446f8e7cc9 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 1,3-Diaminopropane LC-ESI-QQ (API3000, Applied Biosystems) 20V, Positive-QTOF | splash10-0a4i-9000000000-ea81063cc5dad3d70e4a | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 1,3-Diaminopropane LC-ESI-QQ (API3000, Applied Biosystems) 30V, Positive-QTOF | splash10-0a4i-9000000000-ae248307978d5a58cad9 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 1,3-Diaminopropane LC-ESI-QQ (API3000, Applied Biosystems) 40V, Positive-QTOF | splash10-0a4l-9100000000-dad850c9eb355f048f2d | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 1,3-Diaminopropane LC-ESI-QQ (API3000, Applied Biosystems) 50V, Positive-QTOF | splash10-0006-9100000000-85942617edb7fb706c9f | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 1,3-Diaminopropane LC-ESI-QQ , positive-QTOF | splash10-004i-9000000000-1d904fa654446f8e7cc9 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 1,3-Diaminopropane LC-ESI-QQ , positive-QTOF | splash10-0a4i-9000000000-ea81063cc5dad3d70e4a | 2017-09-14 | HMDB team, MONA | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 1,3-Diaminopropane 10V, Positive-QTOF | splash10-056r-9000000000-828d00784702e3b9ed72 | 2015-05-26 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 1,3-Diaminopropane 20V, Positive-QTOF | splash10-0a6u-9000000000-f15f05d0b854daa82e4e | 2015-05-26 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 1,3-Diaminopropane 40V, Positive-QTOF | splash10-0006-9000000000-a218e83369069917d64f | 2015-05-26 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 1,3-Diaminopropane 10V, Positive-QTOF | splash10-056r-9000000000-828d00784702e3b9ed72 | 2015-05-26 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 1,3-Diaminopropane 20V, Positive-QTOF | splash10-0a6u-9000000000-f15f05d0b854daa82e4e | 2015-05-26 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 1,3-Diaminopropane 40V, Positive-QTOF | splash10-0006-9000000000-a218e83369069917d64f | 2015-05-26 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 1,3-Diaminopropane 10V, Negative-QTOF | splash10-00di-9000000000-ade35fabc5f3292f6937 | 2015-05-27 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 1,3-Diaminopropane 20V, Negative-QTOF | splash10-00di-9000000000-9219bb3ff1da1a33cf92 | 2015-05-27 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 1,3-Diaminopropane 40V, Negative-QTOF | splash10-0596-9000000000-67ebedbf9c51f6304af6 | 2015-05-27 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 1,3-Diaminopropane 10V, Negative-QTOF | splash10-00di-9000000000-ade35fabc5f3292f6937 | 2015-05-27 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 1,3-Diaminopropane 20V, Negative-QTOF | splash10-00di-9000000000-9219bb3ff1da1a33cf92 | 2015-05-27 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 1,3-Diaminopropane 40V, Negative-QTOF | splash10-0596-9000000000-67ebedbf9c51f6304af6 | 2015-05-27 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Experimental 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, experimental) | 2012-12-04 | Wishart Lab | View Spectrum | | Experimental 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | 2012-12-04 | Wishart Lab | View Spectrum |
IR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M-H]-) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+H]+) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+Na]+) | 2023-02-03 | FELIX lab | View Spectrum |
|
|---|
| General References | - van den Berg GA, Muskiet FA, Kingma AW, van der Slik W, Halie MR: Simultaneous gas-chromatographic determination of free and acetyl-conjugated polyamines in urine. Clin Chem. 1986 Oct;32(10):1930-7. [PubMed:3757213 ]
- Kim KR, Paik MJ, Kim JH, Dong SW, Jeong DH: Rapid gas chromatographic profiling and screening of biologically active amines. J Pharm Biomed Anal. 1997 Jun;15(9-10):1309-18. [PubMed:9226558 ]
- Bremer HJ, Kohne E: The excretion of diamines in human urine. II. Cadaverine, putrescine, 1,3-diaminopropane, 2,2'-dithiobis(ethylamine) and spermidine in urine of patients with cystinuria and cystinlysinuria. Clin Chim Acta. 1971 May;32(3):407-18. [PubMed:5096953 ]
- Fleisher JH, Russell DH: Estimation of urinary diamines and polyamines by thin-layer chromatography. J Chromatogr. 1975 Jul 16;110(2):335-40. [PubMed:1150841 ]
- Blackburn P, Peterson CM: Thiol-disulfide interchange between cystine and N-2-mercaptoethyl-1, 3-diaminopropane as a potential treatment for cystinuria. Anal Biochem. 1984 Jan;136(1):31-8. [PubMed:6324613 ]
- Aigner-Held R, Campbell RA, Daves GD Jr: Polyamine-pyridoxal Schiff bases in urine. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6652-5. [PubMed:293750 ]
- Somayaji VV, Guay V, Peng Z, Sykes TR, Noujaim AA: Synthesis and evaluation of a new bifunctional chelating agent for the preparation of radioimmunoconjugates. Q J Nucl Med. 1995 Dec;39(4):300-10. [PubMed:8624793 ]
- van den Berg GA, Schaaf JM, Nagel GT, Teelken AW, Muskiet FA: Determination of polyamines and metabolites in cerebrospinal fluid by isotope dilution mass fragmentography, and a clinical application. Clin Chim Acta. 1987 Jun 15;165(2-3):147-54. [PubMed:3308180 ]
- Muskiet FA, van den Berg GA, Kingma AW, Fremouw-Ottevangers DC, Halie MR: Total polyamines and their non-alpha-amino acid metabolites simultaneously determined in urine by capillary gas chromatography, with nitrogen-phosphorus detector; and some clinical applications. Clin Chem. 1984 May;30(5):687-95. [PubMed:6713628 ]
- Lee SH, Suh JW, Chung BC, Kim SO: Polyamine profiles in the urine of patients with leukemia. Cancer Lett. 1998 Jan 9;122(1-2):1-8. [PubMed:9464484 ]
- Thiele I, Swainston N, Fleming RM, Hoppe A, Sahoo S, Aurich MK, Haraldsdottir H, Mo ML, Rolfsson O, Stobbe MD, Thorleifsson SG, Agren R, Bolling C, Bordel S, Chavali AK, Dobson P, Dunn WB, Endler L, Hala D, Hucka M, Hull D, Jameson D, Jamshidi N, Jonsson JJ, Juty N, Keating S, Nookaew I, Le Novere N, Malys N, Mazein A, Papin JA, Price ND, Selkov E Sr, Sigurdsson MI, Simeonidis E, Sonnenschein N, Smallbone K, Sorokin A, van Beek JH, Weichart D, Goryanin I, Nielsen J, Westerhoff HV, Kell DB, Mendes P, Palsson BO: A community-driven global reconstruction of human metabolism. Nat Biotechnol. 2013 May;31(5):419-25. doi: 10.1038/nbt.2488. Epub 2013 Mar 3. [PubMed:23455439 ]
|
|---|