| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected and Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2021-09-14 14:58:04 UTC |
|---|
| HMDB ID | HMDB0001248 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | FAD |
|---|
| Description | Flavin adenine dinucleotide (FAD) is a redox-active coenzyme associated with various proteins, which is involved with several enzymatic reactions in metabolism. FAD, also known as adeflavin or flamitajin b, belongs to the class of organic compounds known as flavin nucleotides. These are nucleotides containing a flavin moiety. Flavin is a compound that contains the tricyclic isoalloxazine ring system, which bears 2 oxo groups at the 2- and 4-positions. FAD is a drug which is used to treat eye diseases caused by vitamin b2 deficiency, such as keratitis and blepharitis. FAD exists in all living species, ranging from bacteria to humans. In humans, FAD is involved in the metabolic disorder called the medium chain acyl-coa dehydrogenase deficiency (mcad) pathway. Outside of the human body, FAD has been detected, but not quantified in several different foods, such as other bread, passion fruits, asparagus, kelps, and green bell peppers. It is a flavoprotein in which the substituent at position 10 of the flavin nucleus is a 5'-adenosyldiphosphoribityl group. |
|---|
| Structure | CC1=CC2=C(C=C1C)N(C[C@H](O)[C@H](O)[C@H](O)COP(O)(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)N1C=NC3=C1N=CN=C3N)C1=NC(=O)NC(=O)C1=N2 InChI=1S/C27H33N9O15P2/c1-10-3-12-13(4-11(10)2)35(24-18(32-12)25(42)34-27(43)33-24)5-14(37)19(39)15(38)6-48-52(44,45)51-53(46,47)49-7-16-20(40)21(41)26(50-16)36-9-31-17-22(28)29-8-30-23(17)36/h3-4,8-9,14-16,19-21,26,37-41H,5-7H2,1-2H3,(H,44,45)(H,46,47)(H2,28,29,30)(H,34,42,43)/t14-,15+,16+,19-,20+,21+,26+/m0/s1 |
|---|
| Synonyms | | Value | Source |
|---|
| Adenosine 5'-(trihydrogen pyrophosphate), 5'-5'-ester with riboflavine | ChEBI | | Adenosine 5'-[3-(riboflavin-5'-yl) dihydrogen diphosphate] | ChEBI | | Flavin adenine dinucleotide | ChEBI | | FLAVIN-adenine dinucleotide | ChEBI | | Riboflavin 5'-(trihydrogen diphosphate), 5'-5'-ester with adenosine | ChEBI | | Riboflavin 5'-adenosine diphosphate | ChEBI | | Adeflavin | Kegg | | Adenosine 5'-(trihydrogen pyrophosphoric acid), 5'-5'-ester with riboflavine | Generator | | Adenosine 5'-[3-(riboflavin-5'-yl) dihydrogen diphosphoric acid] | Generator | | Riboflavin 5'-(trihydrogen diphosphoric acid), 5'-5'-ester with adenosine | Generator | | Riboflavin 5'-adenosine diphosphoric acid | Generator | | 1H-Purin-6-amine flavin dinucleotide | HMDB | | 1H-Purin-6-amine flavine dinucleotide | HMDB | | Adenine-flavin dinucleotide | HMDB | | Adenine-flavine dinucleotide | HMDB | | Adenine-riboflavin dinuceotide | HMDB | | Adenine-riboflavin dinucleotide | HMDB | | Adenine-riboflavine dinucleotide | HMDB | | Flamitajin b | HMDB | | Flanin F | HMDB | | Flavin adenine dinucleotide oxidized | HMDB | | Flavine adenosine diphosphate | HMDB | | Flavine-adenine dinucleotide | HMDB | | Flavitan | HMDB | | Flaziren | HMDB | | Isoalloxazine-adenine dinucleotide | HMDB | | Riboflavin-adenine dinucleotide | HMDB | | Riboflavine-adenine dinucleotide | HMDB | | Dinucleotide, flavin-adenine | HMDB |
|
|---|
| Chemical Formula | C27H33N9O15P2 |
|---|
| Average Molecular Weight | 785.5497 |
|---|
| Monoisotopic Molecular Weight | 785.157134455 |
|---|
| IUPAC Name | {[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy}[({[(2R,3S,4S)-5-{7,8-dimethyl-2,4-dioxo-2H,3H,4H,10H-benzo[g]pteridin-10-yl}-2,3,4-trihydroxypentyl]oxy}(hydroxy)phosphoryl)oxy]phosphinic acid |
|---|
| Traditional Name | flavine-adenine dinucleotide |
|---|
| CAS Registry Number | 146-14-5 |
|---|
| SMILES | CC1=CC2=C(C=C1C)N(C[C@H](O)[C@H](O)[C@H](O)COP(O)(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)N1C=NC3=C1N=CN=C3N)C1=NC(=O)NC(=O)C1=N2 |
|---|
| InChI Identifier | InChI=1S/C27H33N9O15P2/c1-10-3-12-13(4-11(10)2)35(24-18(32-12)25(42)34-27(43)33-24)5-14(37)19(39)15(38)6-48-52(44,45)51-53(46,47)49-7-16-20(40)21(41)26(50-16)36-9-31-17-22(28)29-8-30-23(17)36/h3-4,8-9,14-16,19-21,26,37-41H,5-7H2,1-2H3,(H,44,45)(H,46,47)(H2,28,29,30)(H,34,42,43)/t14-,15+,16+,19-,20+,21+,26+/m0/s1 |
|---|
| InChI Key | VWWQXMAJTJZDQX-UYBVJOGSSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as flavin nucleotides. These are nucleotides containing a flavin moiety. Flavin is a compound that contains the tricyclic isoalloxazine ring system, which bears 2 oxo groups at the 2- and 4-positions. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Nucleosides, nucleotides, and analogues |
|---|
| Class | Flavin nucleotides |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Flavin nucleotides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Flavin nucleotide
- (3'->5')-dinucleotide
- (3'->5')-dinucleotide or analogue
- Purine ribonucleoside diphosphate
- Purine ribonucleoside monophosphate
- Flavin
- Isoalloxazine
- Pentose-5-phosphate
- Pentose phosphate
- Glycosyl compound
- N-glycosyl compound
- Diazanaphthalene
- Pentose monosaccharide
- Pteridine
- 6-aminopurine
- Quinoxaline
- Organic pyrophosphate
- Monosaccharide phosphate
- Imidazopyrimidine
- Purine
- Monoalkyl phosphate
- Aminopyrimidine
- Pyrimidone
- Monosaccharide
- N-substituted imidazole
- Organic phosphoric acid derivative
- Imidolactam
- Benzenoid
- Alkyl phosphate
- Phosphoric acid ester
- Pyrimidine
- Pyrazine
- Tetrahydrofuran
- Azole
- Vinylogous amide
- Heteroaromatic compound
- Imidazole
- Secondary alcohol
- Lactam
- Polyol
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Organic oxide
- Organic oxygen compound
- Organopnictogen compound
- Organonitrogen compound
- Hydrocarbon derivative
- Primary amine
- Alcohol
- Amine
- Organic nitrogen compound
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | |
|---|
| Disposition | |
|---|
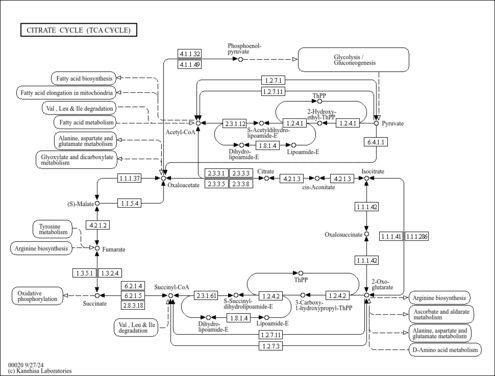
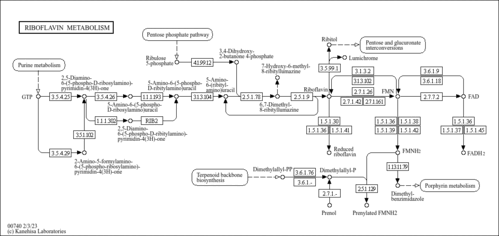
| Process | Naturally occurring processBiological processBiochemical pathwayMetabolic pathway- Ethylmalonic encephalopathy (HMDB: HMDB0001248)
Disease pathway- Prolidase Deficiency (PD) (PathBank: SMP0000207)
- beta-Ketothiolase Deficiency (PathBank: SMP0000173)
- 2-Methyl-3-hydroxybutryl-CoA Dehydrogenase Deficiency (PathBank: SMP0000137)
- Propionic Acidemia (PathBank: SMP0000236)
- 3-Hydroxy-3-methylglutaryl-CoA Lyase Deficiency (PathBank: SMP0000138)
- Maple Syrup Urine Disease (PathBank: SMP0000199)
- 3-Methylcrotonyl-CoA Carboxylase Deficiency Type I (PathBank: SMP0000237)
- 3-Methylglutaconic Aciduria Type I (PathBank: SMP0000139)
- 3-Methylglutaconic Aciduria Type III (PathBank: SMP0000140)
- Methylmalonate Semialdehyde Dehydrogenase Deficiency (PathBank: SMP0000384)
- Methylmalonic Aciduria (PathBank: SMP0000200)
- 4-Hydroxybutyric Aciduria/Succinic Semialdehyde Dehydrogenase Deficiency (PathBank: SMP0000243)
- Homocarnosinosis (PathBank: SMP0000385)
- Hyperinsulinism-Hyperammonemia Syndrome (PathBank: SMP0000339)
- Glutathione Synthetase Deficiency (PathBank: SMP0000337)
- 5-Oxoprolinuria (PathBank: SMP0000143)
- Adenosine Deaminase Deficiency (PathBank: SMP0000144)
- Adenylosuccinate Lyase Deficiency (PathBank: SMP0000167)
- Gout or Kelley-Seegmiller Syndrome (PathBank: SMP0000365)
- Lesch-Nyhan Syndrome (LNS) (PathBank: SMP0000364)
- Molybdenum Cofactor Deficiency (PathBank: SMP0000203)
- Xanthine Dehydrogenase Deficiency (Xanthinuria) (PathBank: SMP0000220)
- Purine Nucleoside Phosphorylase Deficiency (PathBank: SMP0000210)
- AICA-Ribosiduria (PathBank: SMP0000168)
- Arginine: Glycine Amidinotransferase Deficiency (AGAT Deficiency) (PathBank: SMP0000362)
- Hyperprolinemia Type II (PathBank: SMP0000360)
- Hyperprolinemia Type I (PathBank: SMP0000361)
- Prolinemia Type II (PathBank: SMP0000208)
- Guanidinoacetate Methyltransferase Deficiency (GAMT Deficiency) (PathBank: SMP0000188)
- Ornithine Aminotransferase Deficiency (OAT Deficiency) (PathBank: SMP0000363)
- Isovaleric Aciduria (PathBank: SMP0000238)
- Canavan Disease (PathBank: SMP0000175)
- Hypoacetylaspartia (PathBank: SMP0000192)
- Smith-Lemli-Opitz Syndrome (SLOS) (PathBank: SMP0000389)
- CHILD Syndrome (PathBank: SMP0000387)
- Desmosterolosis (PathBank: SMP0000386)
- Chondrodysplasia Punctata II, X-Linked Dominant (CDPX2) (PathBank: SMP0000388)
- Lysosomal Acid Lipase Deficiency (Wolman Disease) (PathBank: SMP0000319)
- Cystathionine beta-Synthase Deficiency (PathBank: SMP0000177)
- Hypermethioninemia (PathBank: SMP0000341)
- S-Adenosylhomocysteine (SAH) Hydrolase Deficiency (PathBank: SMP0000214)
- Glycine N-Methyltransferase Deficiency (PathBank: SMP0000222)
- Methylenetetrahydrofolate Reductase Deficiency (MTHFRD) (PathBank: SMP0000340)
- Methionine Adenosyltransferase Deficiency (PathBank: SMP0000221)
- Ethylmalonic Encephalopathy (PathBank: SMP0000181)
- Glutaric Aciduria Type I (PathBank: SMP0000185)
- Short-Chain Acyl-CoA Dehydrogenase Deficiency (SCAD Deficiency) (PathBank: SMP0000235)
- GABA-Transaminase Deficiency (PathBank: SMP0000351)
- gamma-Glutamyltransferase Deficiency (PathBank: SMP0000183)
- Saccharopinuria/Hyperlysinemia II (PathBank: SMP0000239)
- Histidinemia (PathBank: SMP0000191)
- Leigh Syndrome (PathBank: SMP0000196)
- Pyruvate Decarboxylase E1 Component Deficiency (PDHE1 Deficiency) (PathBank: SMP0000334)
- Pyruvate Dehydrogenase Complex Deficiency (PathBank: SMP0000212)
- Phenylketonuria (PathBank: SMP0000206)
- Tyrosinemia Type 2 (or Richner-Hanhart Syndrome) (PathBank: SMP0000369)
- Tyrosinemia Type 3 (TYRO3) (PathBank: SMP0000370)
- Acute Intermittent Porphyria (PathBank: SMP0000344)
- Porphyria Variegata (PV) (PathBank: SMP0000346)
- Congenital Erythropoietic Porphyria (CEP) or Gunther Disease (PathBank: SMP0000345)
- Alkaptonuria (PathBank: SMP0000169)
- Hawkinsinuria (PathBank: SMP0000190)
- Tyrosinemia Type I (PathBank: SMP0000218)
- beta-Ureidopropionase Deficiency (PathBank: SMP0000172)
- UMP Synthase Deficiency (Orotic Aciduria) (PathBank: SMP0000219)
- Dihydropyrimidinase Deficiency (PathBank: SMP0000178)
- MNGIE (Mitochondrial Neurogastrointestinal Encephalopathy) (PathBank: SMP0000202)
- Congenital Bile Acid Synthesis Defect Type II (PathBank: SMP0000314)
- Congenital Bile Acid Synthesis Defect Type III (PathBank: SMP0000318)
- Familial Hypercholanemia (FHCA) (PathBank: SMP0000317)
- Zellweger Syndrome (PathBank: SMP0000316)
- Cerebrotendinous Xanthomatosis (CTX) (PathBank: SMP0000315)
- Dimethylglycine Dehydrogenase Deficiency (PathBank: SMP0000242)
- Dihydropyrimidine Dehydrogenase Deficiency (DHPD) (PathBank: SMP0000179)
- Sarcosinemia (PathBank: SMP0000244)
- Glycerol Kinase Deficiency (PathBank: SMP0000187)
- Malonic Aciduria (PathBank: SMP0000198)
- Methylmalonic Aciduria Due to Cobalamin-Related Disorders (PathBank: SMP0000201)
- Non-Ketotic Hyperglycinemia (PathBank: SMP0000223)
- Hereditary Coproporphyria (HCP) (PathBank: SMP0000342)
- 2-Hydroxyglutric Aciduria (D and L Form) (PathBank: SMP0000136)
- 3-Methylglutaconic Aciduria Type IV (PathBank: SMP0000141)
- Hypercholesterolemia (PathBank: SMP0000209)
- Hyperglycinemia, Non-Ketotic (PathBank: SMP0000485)
- Carnosinuria, Carnosinemia (PathBank: SMP0000493)
- Tyrosinemia, Transient, of the Newborn (PathBank: SMP0000494)
- Dopamine beta-Hydroxylase Deficiency (PathBank: SMP0000498)
- 5-Oxoprolinase Deficiency (PathBank: SMP0000500)
- gamma-Glutamyltranspeptidase Deficiency (PathBank: SMP0000501)
- Malonyl-CoA Decarboxylase Deficiency (PathBank: SMP0000502)
- Hypophosphatasia (PathBank: SMP0000503)
- Creatine Deficiency, Guanidinoacetate Methyltransferase Deficiency (PathBank: SMP0000504)
- Hyperornithinemia with Gyrate Atrophy (HOGA) (PathBank: SMP0000505)
- Hyperornithinemia-Hyperammonemia-Homocitrullinuria [HHH-syndrome] (PathBank: SMP0000506)
- L-Arginine:Glycine Amidinotransferase Deficiency (PathBank: SMP0000507)
- Cholesteryl Ester Storage Disease (PathBank: SMP0000508)
- Hyper-IgD Syndrome (PathBank: SMP0000509)
- Mevalonic Aciduria (PathBank: SMP0000510)
- Wolman Disease (PathBank: SMP0000511)
- Xanthinuria Type I (PathBank: SMP0000512)
- Xanthinuria Type II (PathBank: SMP0000513)
- 3-Hydroxyisobutyric Acid Dehydrogenase Deficiency (PathBank: SMP0000521)
- 3-Hydroxyisobutyric Aciduria (PathBank: SMP0000522)
- Isobutyryl-CoA Dehydrogenase Deficiency (PathBank: SMP0000523)
- Isovaleric Acidemia (PathBank: SMP0000524)
- Hyperlysinemia I, Familial (PathBank: SMP0000527)
- Hyperlysinemia II or Saccharopinuria (PathBank: SMP0000528)
- D-Glyceric Acidura (PathBank: SMP0000529)
- Familial Lipoprotein Lipase Deficiency (PathBank: SMP0000530)
- Monoamine Oxidase-A Deficiency (MAO-A) (PathBank: SMP0000533)
- Adenine Phosphoribosyltransferase Deficiency (APRT) (PathBank: SMP0000535)
- Mitochondrial DNA Depletion Syndrome (PathBank: SMP0000536)
- Myoadenylate Deaminase Deficiency (PathBank: SMP0000537)
- Carnitine Palmitoyl Transferase Deficiency I (PathBank: SMP0000538)
- Long Chain Acyl-CoA Dehydrogenase Deficiency (LCAD) (PathBank: SMP0000539)
- Very-Long-Chain Acyl-CoA Dehydrogenase Deficiency (VLCAD) (PathBank: SMP0000540)
- Carnitine Palmitoyl Transferase Deficiency II (PathBank: SMP0000541)
- Medium Chain Acyl-CoA Dehydrogenase Deficiency (MCAD) (PathBank: SMP0000542)
- Trifunctional Protein Deficiency (PathBank: SMP0000545)
- Congenital Lactic Acidosis (PathBank: SMP0000546)
- Fumarase Deficiency (PathBank: SMP0000547)
- Mitochondrial Complex II Deficiency (PathBank: SMP0000548)
- 2-Ketoglutarate Dehydrogenase Complex Deficiency (PathBank: SMP0000549)
- Pyruvate Dehydrogenase Deficiency (E3) (PathBank: SMP0000550)
- Pyruvate Dehydrogenase Deficiency (E2) (PathBank: SMP0000551)
- Primary Hyperoxaluria II, PH2 (PathBank: SMP0000558)
- Pyruvate Kinase Deficiency (PathBank: SMP0000559)
- Succinic Semialdehyde Dehydrogenase Deficiency (PathBank: SMP0000567)
- Short-Chain 3-Hydroxyacyl-CoA Dehydrogenase Deficiency (HADH) (PathBank: SMP0000568)
- Homocystinuria-Megaloblastic Anemia Due to Defect in Cobalamin Metabolism, cblG Complementation Type (PathBank: SMP0000570)
- Pyridoxine Dependency with Seizures (PathBank: SMP0000571)
- 2-Aminoadipic 2-Oxoadipic Aciduria (PathBank: SMP0000719)
- 27-Hydroxylase Deficiency (PathBank: SMP0000720)
- 3-Phosphoglycerate Dehydrogenase Deficiency (PathBank: SMP0000721)
- Folate Malabsorption, Hereditary (PathBank: SMP0000724)
- The Oncogenic Action of 2-Hydroxyglutarate (PathBank: SMP0002291)
- The Oncogenic Action of Succinate (PathBank: SMP0002292)
- The Oncogenic Action of Fumarate (PathBank: SMP0002295)
- Glutaminolysis and Cancer (PathBank: SMP0002298)
- Sarcosine Oncometabolite Pathway (PathBank: SMP0002313)
- The Oncogenic Action of L-2-Hydroxyglutarate in Hydroxygluaricaciduria (PathBank: SMP0002358)
- The Oncogenic Action of D-2-Hydroxyglutarate in Hydroxygluaricaciduria (PathBank: SMP0002359)
- 2-Methyl-3-hydroxybutryl-CoA Dehydrogenase Deficiency (PathBank: SMP0120439)
- Hyperornithinemia-Hyperammonemia-Homocitrullinuria [HHH-syndrome] (PathBank: SMP0120571)
- Mitochondrial DNA Depletion Syndrome (PathBank: SMP0120601)
- Short-Chain 3-Hydroxyacyl-CoA Dehydrogenase Deficiency (SCHAD) (PathBank: SMP0120632)
Drug action pathwaySignaling pathwayProtein pathway Biochemical process |
|---|
| Role | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 5 mg/mL | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Experimental Collision Cross Sections |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Kovats Retention IndicesUnderivatized |
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - FAD GC-MS (Non-derivatized) - 70eV, Positive | splash10-0170-1032960400-cdd3286ebb1dea7c4f54 | 2017-11-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - FAD GC-MS (TMS_1_1) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - FAD GC-MS (TMS_1_2) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - FAD GC-MS (TMS_1_3) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - FAD GC-MS (TMS_1_4) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - FAD GC-MS (TMS_1_5) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - FAD GC-MS (TMS_1_6) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - FAD GC-MS (TMS_1_7) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - FAD GC-MS (TMS_1_8) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - FAD GC-MS (TMS_1_9) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - FAD GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - FAD GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - FAD GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - FAD GC-MS (TBDMS_1_4) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - FAD GC-MS (TBDMS_1_5) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - FAD GC-MS (TBDMS_1_6) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - FAD GC-MS (TBDMS_1_7) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - FAD GC-MS (TBDMS_1_8) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - FAD GC-MS (TBDMS_1_9) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental LC-MS/MS | LC-MS/MS Spectrum - FAD Quattro_QQQ 10V, Positive-QTOF (Annotated) | splash10-000i-0001200900-b0740b3d33d50996ccde | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - FAD Quattro_QQQ 25V, Positive-QTOF (Annotated) | splash10-000m-0105900000-3abff26d5bb35f8527b8 | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - FAD Quattro_QQQ 40V, Positive-QTOF (Annotated) | splash10-000i-0931700000-73f360589a13230eea7a | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - FAD LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive-QTOF | splash10-000j-0908600300-b1eccda8ebae8b9e4cc1 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - FAD LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive-QTOF | splash10-000i-0900000000-bdb826f9c3cbc09eff9b | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - FAD LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive-QTOF | splash10-00di-0019800000-00ad56b6b6a3bb4516e6 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - FAD LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive-QTOF | splash10-000b-0009400000-667064ca470a5c341974 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - FAD LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive-QTOF | splash10-000j-0509700500-8766cb874f927ed5a795 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - FAD LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive-QTOF | splash10-000i-0900000000-3125e04c09a14c62f22a | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - FAD LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive-QTOF | splash10-00di-0019700000-78c46ea4b4f562757e56 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - FAD LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive-QTOF | splash10-000b-0009400000-94b845d2c8d082b9484c | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - FAD LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative-QTOF | splash10-001i-0000100900-6bac7b7f631dfc074a0c | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - FAD LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative-QTOF | splash10-001i-0920000000-49c9d4fb9b57a59f45a5 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - FAD LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative-QTOF | splash10-000i-0003900000-572f0b1bd59f71ab3c5f | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - FAD LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative-QTOF | splash10-000i-0003900000-d2ede6a2e7183f1c32a3 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - FAD LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative-QTOF | splash10-001i-0000100900-78554afbc26abe26af35 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - FAD LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative-QTOF | splash10-001i-0930000000-274da0a01c651b791733 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - FAD LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative-QTOF | splash10-000i-0003900000-55939cda9e14fc582757 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - FAD LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative-QTOF | splash10-000i-0003900000-db784762434fac3d351f | 2012-08-31 | HMDB team, MONA | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - FAD 10V, Positive-QTOF | splash10-000i-0932110400-f05626487523221c34de | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - FAD 20V, Positive-QTOF | splash10-000i-0930000000-86c3d6490bc3a27e053a | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - FAD 40V, Positive-QTOF | splash10-052r-0980000000-58a46c839e0e520474d7 | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - FAD 10V, Negative-QTOF | splash10-0006-8482400900-2c4839849821d2807c57 | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - FAD 20V, Negative-QTOF | splash10-001l-5920100000-b38889aa3d09576e2f3e | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - FAD 40V, Negative-QTOF | splash10-0a7l-3900000000-f7144f6355fab252a516 | 2016-09-12 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Experimental 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | 2012-12-05 | Wishart Lab | View Spectrum |
|
|---|
| General References | - Flatz G, Simmersbach F: Flavin adenine dinucleotide concentration in erythrocytes with normal and deficient glucose-6-phosphate dehydrogenase. Klin Wochenschr. 1970 Sep 1;48(17):1071-2. [PubMed:5523465 ]
- Zempleni J: Determination of riboflavin and flavocoenzymes in human blood plasma by high-performance liquid chromatography. Ann Nutr Metab. 1995;39(4):224-6. [PubMed:8546438 ]
- Becker K, Wilkinson AR: Flavin adenine dinucleotide levels in erythrocytes of very low birthweight infants under vitamin supplementation. Biol Neonate. 1993;63(2):80-5. [PubMed:8448258 ]
- Lisowsky T, Lee JE, Polimeno L, Francavilla A, Hofhaus G: Mammalian augmenter of liver regeneration protein is a sulfhydryl oxidase. Dig Liver Dis. 2001 Mar;33(2):173-80. [PubMed:11346147 ]
- Cimino JA, Jhangiani S, Schwartz E, Cooperman JM: Riboflavin metabolism in the hypothyroid human adult. Proc Soc Exp Biol Med. 1987 Feb;184(2):151-3. [PubMed:3809170 ]
- Kodentsova VM, Vrzhesinskaia OA, Alekseeva IA, Spirichev VB: [Comparison of biochemical criteria for supplying the human body with riboflavin]. Vopr Med Khim. 1991 Sep-Oct;37(5):76-9. [PubMed:1759408 ]
- Lopez-Anaya A, Mayersohn M: Quantification of riboflavin, riboflavin 5'-phosphate and flavin adenine dinucleotide in plasma and urine by high-performance liquid chromatography. J Chromatogr. 1987 Dec 25;423:105-13. [PubMed:3443641 ]
- Van Binsbergen CJ, Odink J, Van den Berg H, Koppeschaar H, Coelingh Bennink HJ: Nutritional status in anorexia nervosa: clinical chemistry, vitamins, iron and zinc. Eur J Clin Nutr. 1988 Nov;42(11):929-37. [PubMed:3074921 ]
- Mohrenweiser HW, Novotny JE: ACP1GUA-1--a low-activity variant of human erythrocyte acid phosphatase: association with increased glutathione reductase activity. Am J Hum Genet. 1982 May;34(3):425-33. [PubMed:7081221 ]
- Gianazza E, Vergani L, Wait R, Brizio C, Brambilla D, Begum S, Giancaspero TA, Conserva F, Eberini I, Bufano D, Angelini C, Pegoraro E, Tramontano A, Barile M: Coordinated and reversible reduction of enzymes involved in terminal oxidative metabolism in skeletal muscle mitochondria from a riboflavin-responsive, multiple acyl-CoA dehydrogenase deficiency patient. Electrophoresis. 2006 Mar;27(5-6):1182-98. [PubMed:16470778 ]
- Cimino JA, Noto RA, Fusco CL, Cooperman JM: Riboflavin metabolism in the hypothyroid newborn. Am J Clin Nutr. 1988 Mar;47(3):481-3. [PubMed:3348160 ]
- Elshenawy S, Pinney SE, Stuart T, Doulias PT, Zura G, Parry S, Elovitz MA, Bennett MJ, Bansal A, Strauss JF 3rd, Ischiropoulos H, Simmons RA: The Metabolomic Signature of the Placenta in Spontaneous Preterm Birth. Int J Mol Sci. 2020 Feb 4;21(3). pii: ijms21031043. doi: 10.3390/ijms21031043. [PubMed:32033212 ]
|
|---|