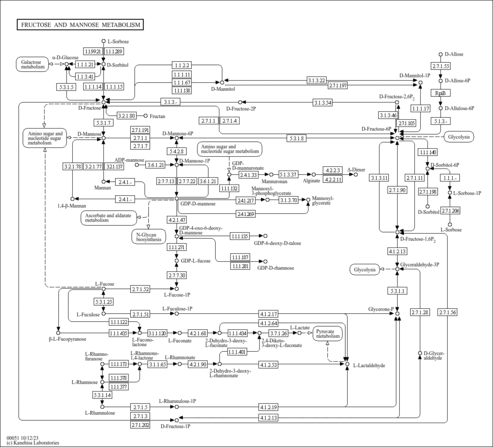
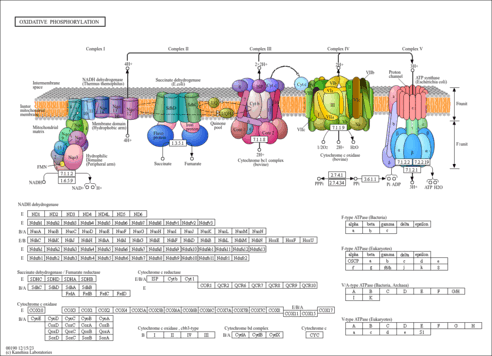
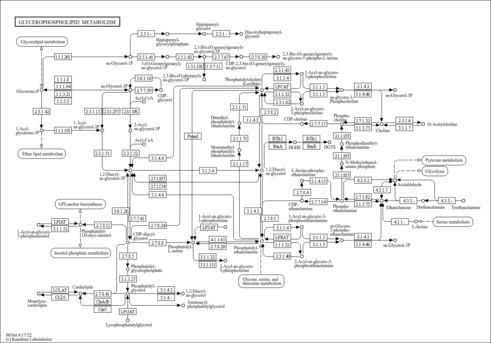
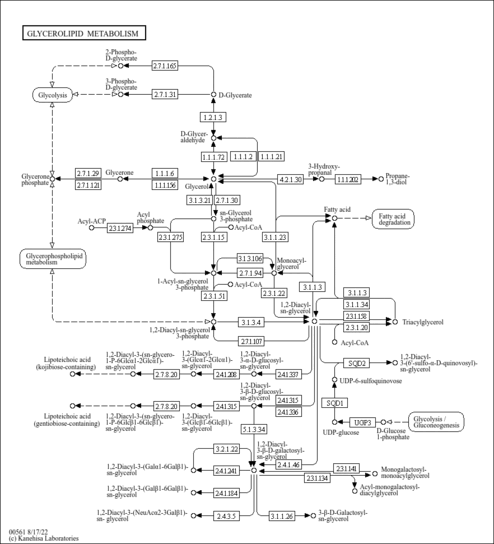
| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| Dihydroxyacetone phosphate,1TMS,isomer #1 | C[Si](C)(C)OCC(=O)COP(=O)(O)O | 1596.3 | Semi standard non polar | 33892256 |
| Dihydroxyacetone phosphate,1TMS,isomer #2 | C[Si](C)(C)OP(=O)(O)OCC(=O)CO | 1600.3 | Semi standard non polar | 33892256 |
| Dihydroxyacetone phosphate,1TMS,isomer #3 | C[Si](C)(C)OC(=COP(=O)(O)O)CO | 1651.5 | Semi standard non polar | 33892256 |
| Dihydroxyacetone phosphate,1TMS,isomer #4 | C[Si](C)(C)OC(=CO)COP(=O)(O)O | 1668.5 | Semi standard non polar | 33892256 |
| Dihydroxyacetone phosphate,2TMS,isomer #1 | C[Si](C)(C)OCC(=O)COP(=O)(O)O[Si](C)(C)C | 1694.9 | Semi standard non polar | 33892256 |
| Dihydroxyacetone phosphate,2TMS,isomer #1 | C[Si](C)(C)OCC(=O)COP(=O)(O)O[Si](C)(C)C | 1611.0 | Standard non polar | 33892256 |
| Dihydroxyacetone phosphate,2TMS,isomer #1 | C[Si](C)(C)OCC(=O)COP(=O)(O)O[Si](C)(C)C | 2082.5 | Standard polar | 33892256 |
| Dihydroxyacetone phosphate,2TMS,isomer #2 | C[Si](C)(C)OCC(=COP(=O)(O)O)O[Si](C)(C)C | 1688.3 | Semi standard non polar | 33892256 |
| Dihydroxyacetone phosphate,2TMS,isomer #2 | C[Si](C)(C)OCC(=COP(=O)(O)O)O[Si](C)(C)C | 1705.8 | Standard non polar | 33892256 |
| Dihydroxyacetone phosphate,2TMS,isomer #2 | C[Si](C)(C)OCC(=COP(=O)(O)O)O[Si](C)(C)C | 2369.6 | Standard polar | 33892256 |
| Dihydroxyacetone phosphate,2TMS,isomer #3 | C[Si](C)(C)OC=C(COP(=O)(O)O)O[Si](C)(C)C | 1751.8 | Semi standard non polar | 33892256 |
| Dihydroxyacetone phosphate,2TMS,isomer #3 | C[Si](C)(C)OC=C(COP(=O)(O)O)O[Si](C)(C)C | 1715.5 | Standard non polar | 33892256 |
| Dihydroxyacetone phosphate,2TMS,isomer #3 | C[Si](C)(C)OC=C(COP(=O)(O)O)O[Si](C)(C)C | 2483.5 | Standard polar | 33892256 |
| Dihydroxyacetone phosphate,2TMS,isomer #4 | C[Si](C)(C)OP(=O)(OCC(=O)CO)O[Si](C)(C)C | 1655.8 | Semi standard non polar | 33892256 |
| Dihydroxyacetone phosphate,2TMS,isomer #4 | C[Si](C)(C)OP(=O)(OCC(=O)CO)O[Si](C)(C)C | 1597.3 | Standard non polar | 33892256 |
| Dihydroxyacetone phosphate,2TMS,isomer #4 | C[Si](C)(C)OP(=O)(OCC(=O)CO)O[Si](C)(C)C | 1897.8 | Standard polar | 33892256 |
| Dihydroxyacetone phosphate,2TMS,isomer #5 | C[Si](C)(C)OC(=CO)COP(=O)(O)O[Si](C)(C)C | 1720.1 | Semi standard non polar | 33892256 |
| Dihydroxyacetone phosphate,2TMS,isomer #5 | C[Si](C)(C)OC(=CO)COP(=O)(O)O[Si](C)(C)C | 1677.4 | Standard non polar | 33892256 |
| Dihydroxyacetone phosphate,2TMS,isomer #5 | C[Si](C)(C)OC(=CO)COP(=O)(O)O[Si](C)(C)C | 2233.0 | Standard polar | 33892256 |
| Dihydroxyacetone phosphate,2TMS,isomer #6 | C[Si](C)(C)OC(=COP(=O)(O)O[Si](C)(C)C)CO | 1700.2 | Semi standard non polar | 33892256 |
| Dihydroxyacetone phosphate,2TMS,isomer #6 | C[Si](C)(C)OC(=COP(=O)(O)O[Si](C)(C)C)CO | 1670.7 | Standard non polar | 33892256 |
| Dihydroxyacetone phosphate,2TMS,isomer #6 | C[Si](C)(C)OC(=COP(=O)(O)O[Si](C)(C)C)CO | 2220.3 | Standard polar | 33892256 |
| Dihydroxyacetone phosphate,3TMS,isomer #1 | C[Si](C)(C)OCC(=O)COP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 1753.6 | Semi standard non polar | 33892256 |
| Dihydroxyacetone phosphate,3TMS,isomer #1 | C[Si](C)(C)OCC(=O)COP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 1676.1 | Standard non polar | 33892256 |
| Dihydroxyacetone phosphate,3TMS,isomer #1 | C[Si](C)(C)OCC(=O)COP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 1779.4 | Standard polar | 33892256 |
| Dihydroxyacetone phosphate,3TMS,isomer #2 | C[Si](C)(C)OCC(=COP(=O)(O)O[Si](C)(C)C)O[Si](C)(C)C | 1762.1 | Semi standard non polar | 33892256 |
| Dihydroxyacetone phosphate,3TMS,isomer #2 | C[Si](C)(C)OCC(=COP(=O)(O)O[Si](C)(C)C)O[Si](C)(C)C | 1706.1 | Standard non polar | 33892256 |
| Dihydroxyacetone phosphate,3TMS,isomer #2 | C[Si](C)(C)OCC(=COP(=O)(O)O[Si](C)(C)C)O[Si](C)(C)C | 1982.0 | Standard polar | 33892256 |
| Dihydroxyacetone phosphate,3TMS,isomer #3 | C[Si](C)(C)OC=C(COP(=O)(O)O[Si](C)(C)C)O[Si](C)(C)C | 1792.8 | Semi standard non polar | 33892256 |
| Dihydroxyacetone phosphate,3TMS,isomer #3 | C[Si](C)(C)OC=C(COP(=O)(O)O[Si](C)(C)C)O[Si](C)(C)C | 1686.2 | Standard non polar | 33892256 |
| Dihydroxyacetone phosphate,3TMS,isomer #3 | C[Si](C)(C)OC=C(COP(=O)(O)O[Si](C)(C)C)O[Si](C)(C)C | 2059.2 | Standard polar | 33892256 |
| Dihydroxyacetone phosphate,3TMS,isomer #4 | C[Si](C)(C)OC(=CO)COP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 1757.3 | Semi standard non polar | 33892256 |
| Dihydroxyacetone phosphate,3TMS,isomer #4 | C[Si](C)(C)OC(=CO)COP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 1756.4 | Standard non polar | 33892256 |
| Dihydroxyacetone phosphate,3TMS,isomer #4 | C[Si](C)(C)OC(=CO)COP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 1890.1 | Standard polar | 33892256 |
| Dihydroxyacetone phosphate,3TMS,isomer #5 | C[Si](C)(C)OC(=COP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)CO | 1739.1 | Semi standard non polar | 33892256 |
| Dihydroxyacetone phosphate,3TMS,isomer #5 | C[Si](C)(C)OC(=COP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)CO | 1724.4 | Standard non polar | 33892256 |
| Dihydroxyacetone phosphate,3TMS,isomer #5 | C[Si](C)(C)OC(=COP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)CO | 1879.4 | Standard polar | 33892256 |
| Dihydroxyacetone phosphate,4TMS,isomer #1 | C[Si](C)(C)OCC(=COP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[Si](C)(C)C | 1810.6 | Semi standard non polar | 33892256 |
| Dihydroxyacetone phosphate,4TMS,isomer #1 | C[Si](C)(C)OCC(=COP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[Si](C)(C)C | 1758.4 | Standard non polar | 33892256 |
| Dihydroxyacetone phosphate,4TMS,isomer #1 | C[Si](C)(C)OCC(=COP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[Si](C)(C)C | 1761.0 | Standard polar | 33892256 |
| Dihydroxyacetone phosphate,4TMS,isomer #2 | C[Si](C)(C)OC=C(COP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[Si](C)(C)C | 1833.6 | Semi standard non polar | 33892256 |
| Dihydroxyacetone phosphate,4TMS,isomer #2 | C[Si](C)(C)OC=C(COP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[Si](C)(C)C | 1740.3 | Standard non polar | 33892256 |
| Dihydroxyacetone phosphate,4TMS,isomer #2 | C[Si](C)(C)OC=C(COP(=O)(O[Si](C)(C)C)O[Si](C)(C)C)O[Si](C)(C)C | 1843.8 | Standard polar | 33892256 |
| Dihydroxyacetone phosphate,1TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OCC(=O)COP(=O)(O)O | 1843.2 | Semi standard non polar | 33892256 |
| Dihydroxyacetone phosphate,1TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OP(=O)(O)OCC(=O)CO | 1852.0 | Semi standard non polar | 33892256 |
| Dihydroxyacetone phosphate,1TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC(=COP(=O)(O)O)CO | 1905.0 | Semi standard non polar | 33892256 |
| Dihydroxyacetone phosphate,1TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)OC(=CO)COP(=O)(O)O | 1888.1 | Semi standard non polar | 33892256 |
| Dihydroxyacetone phosphate,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OCC(=O)COP(=O)(O)O[Si](C)(C)C(C)(C)C | 2109.2 | Semi standard non polar | 33892256 |
| Dihydroxyacetone phosphate,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OCC(=O)COP(=O)(O)O[Si](C)(C)C(C)(C)C | 2052.8 | Standard non polar | 33892256 |
| Dihydroxyacetone phosphate,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OCC(=O)COP(=O)(O)O[Si](C)(C)C(C)(C)C | 2316.7 | Standard polar | 33892256 |
| Dihydroxyacetone phosphate,2TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OCC(=COP(=O)(O)O)O[Si](C)(C)C(C)(C)C | 2161.1 | Semi standard non polar | 33892256 |
| Dihydroxyacetone phosphate,2TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OCC(=COP(=O)(O)O)O[Si](C)(C)C(C)(C)C | 2093.4 | Standard non polar | 33892256 |
| Dihydroxyacetone phosphate,2TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OCC(=COP(=O)(O)O)O[Si](C)(C)C(C)(C)C | 2539.7 | Standard polar | 33892256 |
| Dihydroxyacetone phosphate,2TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC=C(COP(=O)(O)O)O[Si](C)(C)C(C)(C)C | 2150.4 | Semi standard non polar | 33892256 |
| Dihydroxyacetone phosphate,2TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC=C(COP(=O)(O)O)O[Si](C)(C)C(C)(C)C | 2132.4 | Standard non polar | 33892256 |
| Dihydroxyacetone phosphate,2TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC=C(COP(=O)(O)O)O[Si](C)(C)C(C)(C)C | 2634.7 | Standard polar | 33892256 |
| Dihydroxyacetone phosphate,2TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)OP(=O)(OCC(=O)CO)O[Si](C)(C)C(C)(C)C | 2101.4 | Semi standard non polar | 33892256 |
| Dihydroxyacetone phosphate,2TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)OP(=O)(OCC(=O)CO)O[Si](C)(C)C(C)(C)C | 2021.1 | Standard non polar | 33892256 |
| Dihydroxyacetone phosphate,2TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)OP(=O)(OCC(=O)CO)O[Si](C)(C)C(C)(C)C | 2137.5 | Standard polar | 33892256 |
| Dihydroxyacetone phosphate,2TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)OC(=CO)COP(=O)(O)O[Si](C)(C)C(C)(C)C | 2156.2 | Semi standard non polar | 33892256 |
| Dihydroxyacetone phosphate,2TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)OC(=CO)COP(=O)(O)O[Si](C)(C)C(C)(C)C | 2114.6 | Standard non polar | 33892256 |
| Dihydroxyacetone phosphate,2TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)OC(=CO)COP(=O)(O)O[Si](C)(C)C(C)(C)C | 2402.7 | Standard polar | 33892256 |
| Dihydroxyacetone phosphate,2TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)OC(=COP(=O)(O)O[Si](C)(C)C(C)(C)C)CO | 2170.6 | Semi standard non polar | 33892256 |
| Dihydroxyacetone phosphate,2TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)OC(=COP(=O)(O)O[Si](C)(C)C(C)(C)C)CO | 2078.6 | Standard non polar | 33892256 |
| Dihydroxyacetone phosphate,2TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)OC(=COP(=O)(O)O[Si](C)(C)C(C)(C)C)CO | 2395.1 | Standard polar | 33892256 |
| Dihydroxyacetone phosphate,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OCC(=O)COP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 2356.5 | Semi standard non polar | 33892256 |
| Dihydroxyacetone phosphate,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OCC(=O)COP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 2255.6 | Standard non polar | 33892256 |
| Dihydroxyacetone phosphate,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OCC(=O)COP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 2142.5 | Standard polar | 33892256 |
| Dihydroxyacetone phosphate,3TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OCC(=COP(=O)(O)O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 2376.9 | Semi standard non polar | 33892256 |
| Dihydroxyacetone phosphate,3TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OCC(=COP(=O)(O)O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 2265.8 | Standard non polar | 33892256 |
| Dihydroxyacetone phosphate,3TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OCC(=COP(=O)(O)O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 2300.8 | Standard polar | 33892256 |
| Dihydroxyacetone phosphate,3TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC=C(COP(=O)(O)O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 2390.6 | Semi standard non polar | 33892256 |
| Dihydroxyacetone phosphate,3TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC=C(COP(=O)(O)O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 2274.9 | Standard non polar | 33892256 |
| Dihydroxyacetone phosphate,3TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC=C(COP(=O)(O)O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 2329.7 | Standard polar | 33892256 |
| Dihydroxyacetone phosphate,3TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)OC(=CO)COP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 2373.9 | Semi standard non polar | 33892256 |
| Dihydroxyacetone phosphate,3TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)OC(=CO)COP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 2317.1 | Standard non polar | 33892256 |
| Dihydroxyacetone phosphate,3TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)OC(=CO)COP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 2213.3 | Standard polar | 33892256 |
| Dihydroxyacetone phosphate,3TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)OC(=COP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)CO | 2360.0 | Semi standard non polar | 33892256 |
| Dihydroxyacetone phosphate,3TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)OC(=COP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)CO | 2255.4 | Standard non polar | 33892256 |
| Dihydroxyacetone phosphate,3TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)OC(=COP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)CO | 2205.0 | Standard polar | 33892256 |
| Dihydroxyacetone phosphate,4TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OCC(=COP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 2564.2 | Semi standard non polar | 33892256 |
| Dihydroxyacetone phosphate,4TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OCC(=COP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 2406.8 | Standard non polar | 33892256 |
| Dihydroxyacetone phosphate,4TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OCC(=COP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 2202.9 | Standard polar | 33892256 |
| Dihydroxyacetone phosphate,4TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OC=C(COP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 2592.2 | Semi standard non polar | 33892256 |
| Dihydroxyacetone phosphate,4TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OC=C(COP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 2402.3 | Standard non polar | 33892256 |
| Dihydroxyacetone phosphate,4TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OC=C(COP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 2243.6 | Standard polar | 33892256 |