| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected and Quantified |
|---|
| Creation Date | 2006-05-22 15:12:12 UTC |
|---|
| Update Date | 2023-02-21 17:16:29 UTC |
|---|
| HMDB ID | HMDB0002825 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Theobromine |
|---|
| Description | Theobromine, or 3,7-Dimethylxanthine, is the principle alkaloid in Theobroma cacao (the cacao bean) and other plants. A xanthine alkaloid that is used as a bronchodilator and as a vasodilator. It has a weaker diuretic activity than theophylline and is also a less powerful stimulant of smooth muscle. It has practically no stimulant effect on the central nervous system. It was formerly used as a diuretic and in the treatment of angina pectoris and hypertension. Theobromine is a bitter alkaloid of the methylxanthine family, which also includes the similar compounds theophylline and caffeine. Despite its name, the compound contains no bromine. Theobromine is derived from Theobroma, the genus of the cacao tree, which is composed of the Greek roots theo ("God") and broma ("food"), meaning "food of the gods". It is the primary alkaloid found in cocoa and chocolate, and is one of the causes for chocolate's mood-elevating effects. The amount found in chocolate is small enough that chocolate can be safely consumed by humans in large quantities, but animals that metabolize theobromine more slowly, such as cats and dogs, can easily consume enough chocolate to cause chocolate poisoning. Theobromine is a stimulant frequently confused with caffeine. Theobromine has very different effects on the human body from caffeine; it is a mild, lasting stimulant with a mood improving effect, whereas caffeine has a strong, immediate effect and increases stress. In medicine, it is used as a diuretic, vasodilator, and myocardial stimulant. There is a possible association between prostate cancer and theobromine. Theobromine is a contributing factor in acid reflux because it relaxes the esophageal sphincter muscle, allowing stomach acid access to the esophagus. |
|---|
| Structure | CN1C=NC2=C1C(O)=NC(=O)N2C InChI=1S/C7H8N4O2/c1-10-3-8-5-4(10)6(12)9-7(13)11(5)2/h3H,1-2H3,(H,9,12,13) |
|---|
| Synonyms | | Value | Source |
|---|
| 3,7-Dihydro-3,7-dimethyl-1H-purine-2,6-dione | ChEBI | | 3,7-Dimethylpurine-2,6-dione | ChEBI | | 3,7-Dimethylxanthine | ChEBI | | Theobromin | ChEBI | | Teobromin | HMDB | | 2,6-Dihydroxy-3,7-dimethyl-purine | HMDB | | 3,7-Dimethyl-xanthine | HMDB | | Diurobromine | HMDB |
|
|---|
| Chemical Formula | C7H8N4O2 |
|---|
| Average Molecular Weight | 180.164 |
|---|
| Monoisotopic Molecular Weight | 180.06472552 |
|---|
| IUPAC Name | 3,7-dimethyl-2,3,6,7-tetrahydro-1H-purine-2,6-dione |
|---|
| Traditional Name | theobromine |
|---|
| CAS Registry Number | 83-67-0 |
|---|
| SMILES | CN1C=NC2=C1C(O)=NC(=O)N2C |
|---|
| InChI Identifier | InChI=1S/C7H8N4O2/c1-10-3-8-5-4(10)6(12)9-7(13)11(5)2/h3H,1-2H3,(H,9,12,13) |
|---|
| InChI Key | YAPQBXQYLJRXSA-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as xanthines. These are purine derivatives with a ketone group conjugated at carbons 2 and 6 of the purine moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Imidazopyrimidines |
|---|
| Sub Class | Purines and purine derivatives |
|---|
| Direct Parent | Xanthines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Xanthine
- 6-oxopurine
- Purinone
- Alkaloid or derivatives
- Pyrimidone
- N-substituted imidazole
- Pyrimidine
- Azole
- Imidazole
- Heteroaromatic compound
- Vinylogous amide
- Lactam
- Urea
- Azacycle
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Not Available | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | 357 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 0.33 mg/mL at 25 °C | Not Available | | LogP | -0.78 | HANSCH,C ET AL. (1995) |
|
|---|
| Experimental Chromatographic Properties | Experimental Collision Cross Sections |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Retention Times Underivatized| Chromatographic Method | Retention Time | Reference |
|---|
| Measured using a Waters Acquity ultraperformance liquid chromatography (UPLC) ethylene-bridged hybrid (BEH) C18 column (100 mm × 2.1 mm; 1.7 μmparticle diameter). Predicted by Afia on May 17, 2022. Predicted by Afia on May 17, 2022. | 2.42 minutes | 32390414 | | Predicted by Siyang on May 30, 2022 | 8.631 minutes | 33406817 | | Predicted by Siyang using ReTip algorithm on June 8, 2022 | 2.23 minutes | 32390414 | | AjsUoB = Accucore 150 Amide HILIC with 10mM Ammonium Formate, 0.1% Formic Acid | 46.7 seconds | 40023050 | | Fem_Long = Waters ACQUITY UPLC HSS T3 C18 with Water:MeOH and 0.1% Formic Acid | 880.0 seconds | 40023050 | | Fem_Lipids = Ascentis Express C18 with (60:40 water:ACN):(90:10 IPA:ACN) and 10mM NH4COOH + 0.1% Formic Acid | 286.8 seconds | 40023050 | | Life_Old = Waters ACQUITY UPLC BEH C18 with Water:(20:80 acetone:ACN) and 0.1% Formic Acid | 83.6 seconds | 40023050 | | Life_New = RP Waters ACQUITY UPLC HSS T3 C18 with Water:(30:70 MeOH:ACN) and 0.1% Formic Acid | 175.8 seconds | 40023050 | | RIKEN = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 57.9 seconds | 40023050 | | Eawag_XBridgeC18 = XBridge C18 3.5u 2.1x50 mm with Water:MeOH and 0.1% Formic Acid | 270.1 seconds | 40023050 | | BfG_NTS_RP1 =Agilent Zorbax Eclipse Plus C18 (2.1 mm x 150 mm, 3.5 um) with Water:ACN and 0.1% Formic Acid | 259.5 seconds | 40023050 | | HILIC_BDD_2 = Merck SeQuant ZIC-HILIC with ACN(0.1% formic acid):water(16 mM ammonium formate) | 102.8 seconds | 40023050 | | UniToyama_Atlantis = RP Waters Atlantis T3 (2.1 x 150 mm, 5 um) with ACN:Water and 0.1% Formic Acid | 588.3 seconds | 40023050 | | BDD_C18 = Hypersil Gold 1.9µm C18 with Water:ACN and 0.1% Formic Acid | 232.7 seconds | 40023050 | | UFZ_Phenomenex = Kinetex Core-Shell C18 2.6 um, 3.0 x 100 mm, Phenomenex with Water:MeOH and 0.1% Formic Acid | 720.1 seconds | 40023050 | | SNU_RIKEN_POS = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 183.3 seconds | 40023050 | | RPMMFDA = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 177.8 seconds | 40023050 | | MTBLS87 = Merck SeQuant ZIC-pHILIC column with ACN:Water and :ammonium carbonate | 612.5 seconds | 40023050 | | KI_GIAR_zic_HILIC_pH2_7 = Merck SeQuant ZIC-HILIC with ACN:Water and 0.1% FA | 227.6 seconds | 40023050 | | Meister zic-pHILIC pH9.3 = Merck SeQuant ZIC-pHILIC column with ACN:Water 5mM NH4Ac pH9.3 and 5mM ammonium acetate in water | 115.7 seconds | 40023050 |
Predicted Kovats Retention IndicesUnderivatizedDerivatized |
|---|
| Spectra |
|---|
| |
|---|
| Biological Properties |
|---|
| Cellular Locations | - Cytoplasm (predicted from logP)
|
|---|
| Biospecimen Locations | - Blood
- Cerebrospinal Fluid (CSF)
- Feces
- Saliva
- Urine
|
|---|
| Tissue Locations | |
|---|
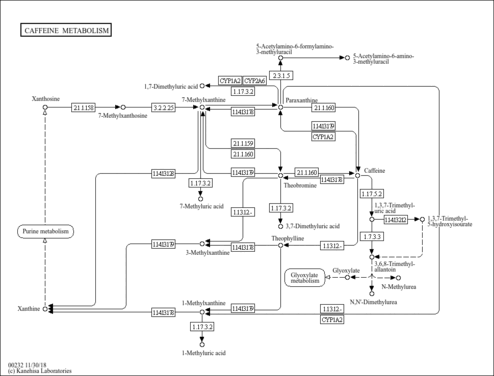
| Pathways | |
|---|
| Normal Concentrations |
|---|
| |
| Blood | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Blood | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Female | Normal | | details | | Blood | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Blood | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Blood | Detected and Quantified | 1.1 +/- 0.2 uM | Adult (>18 years old) | Male | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Not Specified | Normal | | details | | Urine | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected and Quantified | 0.5 (0.36-0.7) umol/mmol creatinine | Adult (>18 years old) | Both | Normal | | details |
|
|---|
| Abnormal Concentrations |
|---|
| |
| Blood | Detected and Quantified | 1.3 +/- 0.5 uM | Adult (>18 years old) | Male | Malaria | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 0.869 +/- 0.182 uM | Adult (>18 years old) | Both | severe traumatic brain injury (TBI) | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 1.710 +/- 0.539 uM | Not Specified | Not Specified | Traumatic Brain Injury (TBI) | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 0.641 +/- 0.169 uM | Not Specified | Not Specified | Traumatic Brain Injury (TBI) | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 0.863 +/- 0.241 uM | Not Specified | Female | Traumatic Brain Injury (TBI) | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 0.886 +/- 0.185 uM | Not Specified | Male | Traumatic Brain Injury (TBI) | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Colorectal Cancer | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Colorectal cancer | | details | | Urine | Detected and Quantified | 3.6 (1.0-7.3) umol/mmol creatinine | Adult (>18 years old) | Both | Asthma | | details | | Urine | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Autosomal dominant polycystic kidney disease (ADPKD) | | details |
|
|---|
| Associated Disorders and Diseases |
|---|
| Disease References | | Malaria |
|---|
- Akinyinka OO, Sowunmi A, Honeywell R, Renwick AG: The effects of acute falciparum malaria on the disposition of caffeine and the comparison of saliva and plasma-derived pharmacokinetic parameters in adult Nigerians. Eur J Clin Pharmacol. 2000 May;56(2):159-65. [PubMed:10877011 ]
| | Head injury |
|---|
- Sachse KT, Jackson EK, Wisniewski SR, Gillespie DG, Puccio AM, Clark RS, Dixon CE, Kochanek PM: Increases in cerebrospinal fluid caffeine concentration are associated with favorable outcome after severe traumatic brain injury in humans. J Cereb Blood Flow Metab. 2008 Feb;28(2):395-401. Epub 2007 Aug 8. [PubMed:17684518 ]
| | Colorectal cancer |
|---|
- Brown DG, Rao S, Weir TL, O'Malia J, Bazan M, Brown RJ, Ryan EP: Metabolomics and metabolic pathway networks from human colorectal cancers, adjacent mucosa, and stool. Cancer Metab. 2016 Jun 6;4:11. doi: 10.1186/s40170-016-0151-y. eCollection 2016. [PubMed:27275383 ]
- Goedert JJ, Sampson JN, Moore SC, Xiao Q, Xiong X, Hayes RB, Ahn J, Shi J, Sinha R: Fecal metabolomics: assay performance and association with colorectal cancer. Carcinogenesis. 2014 Sep;35(9):2089-96. doi: 10.1093/carcin/bgu131. Epub 2014 Jul 18. [PubMed:25037050 ]
| | Asthma |
|---|
- Zydron M, Baranowski J, Baranowska I: Separation, pre-concentration, and HPLC analysis of methylxanthines in urine samples. J Sep Sci. 2004 Oct;27(14):1166-72. [PubMed:15537072 ]
| | Autosomal dominant polycystic kidney disease |
|---|
- Gronwald W, Klein MS, Zeltner R, Schulze BD, Reinhold SW, Deutschmann M, Immervoll AK, Boger CA, Banas B, Eckardt KU, Oefner PJ: Detection of autosomal dominant polycystic kidney disease by NMR spectroscopic fingerprinting of urine. Kidney Int. 2011 Jun;79(11):1244-53. doi: 10.1038/ki.2011.30. Epub 2011 Mar 9. [PubMed:21389975 ]
|
|
|---|
| Associated OMIM IDs | |
|---|
| External Links |
|---|
| DrugBank ID | DB01412 |
|---|
| Phenol Explorer Compound ID | Not Available |
|---|
| FooDB ID | FDB000455 |
|---|
| KNApSAcK ID | C00001509 |
|---|
| Chemspider ID | 5236 |
|---|
| KEGG Compound ID | C07480 |
|---|
| BioCyc ID | 3-7-DIMETHYLXANTHINE |
|---|
| BiGG ID | Not Available |
|---|
| Wikipedia Link | Theobromine |
|---|
| METLIN ID | 1456 |
|---|
| PubChem Compound | 5429 |
|---|
| PDB ID | Not Available |
|---|
| ChEBI ID | 28946 |
|---|
| Food Biomarker Ontology | Not Available |
|---|
| VMH ID | Not Available |
|---|
| MarkerDB ID | MDB00000403 |
|---|
| Good Scents ID | rw1036961 |
|---|
| References |
|---|
| Synthesis Reference | Yoon, Hye-Sung; Hwang, In-Gyun; Bang, Won-Gi. Production of theobromine from caffeine by Pseudomonas sp. Chayon Chawon Nonjip (1995), 35(1), 33-39. |
|---|
| Material Safety Data Sheet (MSDS) | Download (PDF) |
|---|
| General References | - Holstege A, Kurz M, Weinbeck M, Gerok W: Excretion of caffeine and its primary degradation products into bile. J Hepatol. 1993 Jan;17(1):67-73. [PubMed:8445222 ]
- Delahunty T, Schoendorfer D: Caffeine demethylation monitoring using a transdermal sweat patch. J Anal Toxicol. 1998 Nov-Dec;22(7):596-600. [PubMed:9847011 ]
- Blanchard J, Weber CW, Shearer LE: Methylxanthine levels in breast milk of lactating women of different ethnic and socioeconomic classes. Biopharm Drug Dispos. 1992 Apr;13(3):187-96. [PubMed:1576327 ]
- Sachse C, Ruschen S, Dettling M, Schley J, Bauer S, Muller-Oerlinghausen B, Roots I, Brockmoller J: Flavin monooxygenase 3 (FMO3) polymorphism in a white population: allele frequencies, mutation linkage, and functional effects on clozapine and caffeine metabolism. Clin Pharmacol Ther. 1999 Oct;66(4):431-8. [PubMed:10546928 ]
- Desiraju RK, Sugita ET, Mayock RL: Determination of theophylline and its metabolites by liquid chromatography. J Chromatogr Sci. 1977 Dec;15(12):563-8. [PubMed:591601 ]
- Tarka SM Jr, Arnaud MJ, Dvorchik BH, Vesell ES: Theobromine kinetics and metabolic disposition. Clin Pharmacol Ther. 1983 Oct;34(4):546-55. [PubMed:6617078 ]
- Tserng KY, King KC, Takieddine FN: Theophylline metabolism in premature infants. Clin Pharmacol Ther. 1981 May;29(5):594-600. [PubMed:7214789 ]
- Gonzalez-Jimenez J, Frutos G, Cayre I: Fluorescence quenching of human serum albumin by xanthines. Biochem Pharmacol. 1992 Aug 18;44(4):824-6. [PubMed:1510729 ]
- Sommer KR, Hill RM, Horning MG: Identification and quantification of drugs in human amniotic fluid. Res Commun Chem Pathol Pharmacol. 1975 Nov;12(3):583-95. [PubMed:1197933 ]
- Skopinska-Rozewska E, Janik P, Przybyszewska M, Sommer E, Bialas-Chromiec B: Inhibitory effect of theobromine on induction of angiogenesis and VEGF mRNA expression in v-raf transfectants of human urothelial cells HCV-29. Int J Mol Med. 1998 Dec;2(6):649-52. [PubMed:9850731 ]
- Resman BH, Blumenthal P, Jusko WJ: Breast milk distribution of theobromine from chocolate. J Pediatr. 1977 Sep;91(3):477-80. [PubMed:894424 ]
- Scott NR, Chakraborty J, Marks V: Determination of caffeine, theophylline and theobromine in serum and saliva using high-performance liquid chromatography. Ann Clin Biochem. 1984 Mar;21 ( Pt 2):120-4. [PubMed:6712142 ]
- Emara S: Simultaneous determination of caffeine, theophylline and theobromine in human plasma by on-line solid-phase extraction coupled to reversed-phase chromatography. Biomed Chromatogr. 2004 Oct;18(8):479-85. [PubMed:15386526 ]
- Gennaro MC, Abrigo C, Biglino P: Quantification of theophylline in human plasma by reversed-phase ion-interaction high-performance liquid chromatography and comparison with the TDx fluorescence polarization immunoassay procedure. Analyst. 1992 Jul;117(7):1071-4. [PubMed:1524227 ]
- Slattery ML, West DW: Smoking, alcohol, coffee, tea, caffeine, and theobromine: risk of prostate cancer in Utah (United States). Cancer Causes Control. 1993 Nov;4(6):559-63. [PubMed:8280834 ]
- Usmani OS, Belvisi MG, Patel HJ, Crispino N, Birrell MA, Korbonits M, Korbonits D, Barnes PJ: Theobromine inhibits sensory nerve activation and cough. FASEB J. 2005 Feb;19(2):231-3. Epub 2004 Nov 17. [PubMed:15548587 ]
- Elshenawy S, Pinney SE, Stuart T, Doulias PT, Zura G, Parry S, Elovitz MA, Bennett MJ, Bansal A, Strauss JF 3rd, Ischiropoulos H, Simmons RA: The Metabolomic Signature of the Placenta in Spontaneous Preterm Birth. Int J Mol Sci. 2020 Feb 4;21(3). pii: ijms21031043. doi: 10.3390/ijms21031043. [PubMed:32033212 ]
|
|---|