| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected but not Quantified |
|---|
| Creation Date | 2008-10-16 22:26:25 UTC |
|---|
| Update Date | 2023-02-21 17:17:27 UTC |
|---|
| HMDB ID | HMDB0011107 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 7-Methyluric acid |
|---|
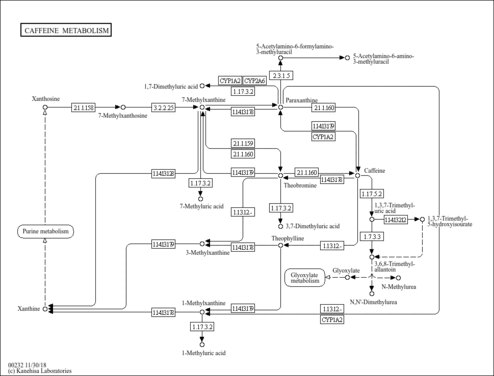
| Description | 7-Methyluric acid, also known as 7-methylate, belongs to the class of organic compounds known as xanthines. These are purine derivatives with a ketone group conjugated at carbons 2 and 6 of the purine moiety. 7-Methyluric acid is the minor urinary metabolites of caffeine. 7-Methyluric acid is an extremely weak basic (essentially neutral) compound (based on its pKa). 7-Methyluric acid exists in all living organisms, ranging from bacteria to humans. 7-methyluric acid can be biosynthesized from 7-methylxanthine through its interaction with the enzyme xanthine dehydrogenase/oxidase. In humans, 7-methyluric acid is involved in caffeine metabolism. |
|---|
| Structure | CN1C(=O)NC2=C1C(=O)NC(=O)N2 InChI=1S/C6H6N4O3/c1-10-2-3(8-6(10)13)7-5(12)9-4(2)11/h1H3,(H3,7,8,9,11,12,13) |
|---|
| Synonyms | | Value | Source |
|---|
| 7-Methylate | Generator | | 7-Methylic acid | Generator |
|
|---|
| Chemical Formula | C6H6N4O3 |
|---|
| Average Molecular Weight | 182.1368 |
|---|
| Monoisotopic Molecular Weight | 182.043990078 |
|---|
| IUPAC Name | 7-methyl-7H-purine-2,6,8-triol |
|---|
| Traditional Name | 7-methyluric acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | CN1C(=O)NC2=C1C(=O)NC(=O)N2 |
|---|
| InChI Identifier | InChI=1S/C6H6N4O3/c1-10-2-3(8-6(10)13)7-5(12)9-4(2)11/h1H3,(H3,7,8,9,11,12,13) |
|---|
| InChI Key | YHNNPKUFPWLTOP-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as xanthines. These are purine derivatives with a ketone group conjugated at carbons 2 and 6 of the purine moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Imidazopyrimidines |
|---|
| Sub Class | Purines and purine derivatives |
|---|
| Direct Parent | Xanthines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Xanthine
- 6-oxopurine
- Purinone
- Alkaloid or derivatives
- Pyrimidone
- N-substituted imidazole
- Pyrimidine
- Azole
- Imidazole
- Heteroaromatic compound
- Vinylogous amide
- Lactam
- Urea
- Azacycle
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Experimental Collision Cross Sections |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Retention Times Underivatized| Chromatographic Method | Retention Time | Reference |
|---|
| Measured using a Waters Acquity ultraperformance liquid chromatography (UPLC) ethylene-bridged hybrid (BEH) C18 column (100 mm × 2.1 mm; 1.7 μmparticle diameter). Predicted by Afia on May 17, 2022. Predicted by Afia on May 17, 2022. | 2.5 minutes | 32390414 | | Predicted by Siyang on May 30, 2022 | 9.2554 minutes | 33406817 | | Predicted by Siyang using ReTip algorithm on June 8, 2022 | 2.73 minutes | 32390414 | | Fem_Long = Waters ACQUITY UPLC HSS T3 C18 with Water:MeOH and 0.1% Formic Acid | 450.9 seconds | 40023050 | | Fem_Lipids = Ascentis Express C18 with (60:40 water:ACN):(90:10 IPA:ACN) and 10mM NH4COOH + 0.1% Formic Acid | 321.4 seconds | 40023050 | | Life_Old = Waters ACQUITY UPLC BEH C18 with Water:(20:80 acetone:ACN) and 0.1% Formic Acid | 62.5 seconds | 40023050 | | Life_New = RP Waters ACQUITY UPLC HSS T3 C18 with Water:(30:70 MeOH:ACN) and 0.1% Formic Acid | 195.6 seconds | 40023050 | | RIKEN = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 42.3 seconds | 40023050 | | Eawag_XBridgeC18 = XBridge C18 3.5u 2.1x50 mm with Water:MeOH and 0.1% Formic Acid | 275.8 seconds | 40023050 | | BfG_NTS_RP1 =Agilent Zorbax Eclipse Plus C18 (2.1 mm x 150 mm, 3.5 um) with Water:ACN and 0.1% Formic Acid | 253.5 seconds | 40023050 | | HILIC_BDD_2 = Merck SeQuant ZIC-HILIC with ACN(0.1% formic acid):water(16 mM ammonium formate) | 417.8 seconds | 40023050 | | UniToyama_Atlantis = RP Waters Atlantis T3 (2.1 x 150 mm, 5 um) with ACN:Water and 0.1% Formic Acid | 596.3 seconds | 40023050 | | BDD_C18 = Hypersil Gold 1.9µm C18 with Water:ACN and 0.1% Formic Acid | 120.0 seconds | 40023050 | | UFZ_Phenomenex = Kinetex Core-Shell C18 2.6 um, 3.0 x 100 mm, Phenomenex with Water:MeOH and 0.1% Formic Acid | 680.2 seconds | 40023050 | | SNU_RIKEN_POS = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 189.7 seconds | 40023050 | | RPMMFDA = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 228.6 seconds | 40023050 | | MTBLS87 = Merck SeQuant ZIC-pHILIC column with ACN:Water and :ammonium carbonate | 693.9 seconds | 40023050 | | KI_GIAR_zic_HILIC_pH2_7 = Merck SeQuant ZIC-HILIC with ACN:Water and 0.1% FA | 306.2 seconds | 40023050 | | Meister zic-pHILIC pH9.3 = Merck SeQuant ZIC-pHILIC column with ACN:Water 5mM NH4Ac pH9.3 and 5mM ammonium acetate in water | 256.3 seconds | 40023050 |
Predicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| 7-Methyluric acid,1TMS,isomer #1 | CN1C(=O)N([Si](C)(C)C)C2=C1C(=O)[NH]C(=O)[NH]2 | 1960.3 | Semi standard non polar | 33892256 | | 7-Methyluric acid,1TMS,isomer #1 | CN1C(=O)N([Si](C)(C)C)C2=C1C(=O)[NH]C(=O)[NH]2 | 2177.1 | Standard non polar | 33892256 | | 7-Methyluric acid,1TMS,isomer #1 | CN1C(=O)N([Si](C)(C)C)C2=C1C(=O)[NH]C(=O)[NH]2 | 2965.9 | Standard polar | 33892256 | | 7-Methyluric acid,1TMS,isomer #2 | CN1C(=O)[NH]C2=C1C(=O)N([Si](C)(C)C)C(=O)[NH]2 | 2033.3 | Semi standard non polar | 33892256 | | 7-Methyluric acid,1TMS,isomer #2 | CN1C(=O)[NH]C2=C1C(=O)N([Si](C)(C)C)C(=O)[NH]2 | 2247.7 | Standard non polar | 33892256 | | 7-Methyluric acid,1TMS,isomer #2 | CN1C(=O)[NH]C2=C1C(=O)N([Si](C)(C)C)C(=O)[NH]2 | 2953.0 | Standard polar | 33892256 | | 7-Methyluric acid,1TMS,isomer #3 | CN1C(=O)[NH]C2=C1C(=O)[NH]C(=O)N2[Si](C)(C)C | 1990.4 | Semi standard non polar | 33892256 | | 7-Methyluric acid,1TMS,isomer #3 | CN1C(=O)[NH]C2=C1C(=O)[NH]C(=O)N2[Si](C)(C)C | 2209.5 | Standard non polar | 33892256 | | 7-Methyluric acid,1TMS,isomer #3 | CN1C(=O)[NH]C2=C1C(=O)[NH]C(=O)N2[Si](C)(C)C | 2914.0 | Standard polar | 33892256 | | 7-Methyluric acid,2TMS,isomer #1 | CN1C(=O)N([Si](C)(C)C)C2=C1C(=O)[NH]C(=O)N2[Si](C)(C)C | 1948.1 | Semi standard non polar | 33892256 | | 7-Methyluric acid,2TMS,isomer #1 | CN1C(=O)N([Si](C)(C)C)C2=C1C(=O)[NH]C(=O)N2[Si](C)(C)C | 2279.3 | Standard non polar | 33892256 | | 7-Methyluric acid,2TMS,isomer #1 | CN1C(=O)N([Si](C)(C)C)C2=C1C(=O)[NH]C(=O)N2[Si](C)(C)C | 2512.2 | Standard polar | 33892256 | | 7-Methyluric acid,2TMS,isomer #2 | CN1C(=O)N([Si](C)(C)C)C2=C1C(=O)N([Si](C)(C)C)C(=O)[NH]2 | 2018.4 | Semi standard non polar | 33892256 | | 7-Methyluric acid,2TMS,isomer #2 | CN1C(=O)N([Si](C)(C)C)C2=C1C(=O)N([Si](C)(C)C)C(=O)[NH]2 | 2254.3 | Standard non polar | 33892256 | | 7-Methyluric acid,2TMS,isomer #2 | CN1C(=O)N([Si](C)(C)C)C2=C1C(=O)N([Si](C)(C)C)C(=O)[NH]2 | 2512.6 | Standard polar | 33892256 | | 7-Methyluric acid,2TMS,isomer #3 | CN1C(=O)[NH]C2=C1C(=O)N([Si](C)(C)C)C(=O)N2[Si](C)(C)C | 2007.9 | Semi standard non polar | 33892256 | | 7-Methyluric acid,2TMS,isomer #3 | CN1C(=O)[NH]C2=C1C(=O)N([Si](C)(C)C)C(=O)N2[Si](C)(C)C | 2271.8 | Standard non polar | 33892256 | | 7-Methyluric acid,2TMS,isomer #3 | CN1C(=O)[NH]C2=C1C(=O)N([Si](C)(C)C)C(=O)N2[Si](C)(C)C | 2535.0 | Standard polar | 33892256 | | 7-Methyluric acid,3TMS,isomer #1 | CN1C(=O)N([Si](C)(C)C)C2=C1C(=O)N([Si](C)(C)C)C(=O)N2[Si](C)(C)C | 2146.7 | Semi standard non polar | 33892256 | | 7-Methyluric acid,3TMS,isomer #1 | CN1C(=O)N([Si](C)(C)C)C2=C1C(=O)N([Si](C)(C)C)C(=O)N2[Si](C)(C)C | 2273.0 | Standard non polar | 33892256 | | 7-Methyluric acid,3TMS,isomer #1 | CN1C(=O)N([Si](C)(C)C)C2=C1C(=O)N([Si](C)(C)C)C(=O)N2[Si](C)(C)C | 2280.2 | Standard polar | 33892256 | | 7-Methyluric acid,1TBDMS,isomer #1 | CN1C(=O)N([Si](C)(C)C(C)(C)C)C2=C1C(=O)[NH]C(=O)[NH]2 | 2137.9 | Semi standard non polar | 33892256 | | 7-Methyluric acid,1TBDMS,isomer #1 | CN1C(=O)N([Si](C)(C)C(C)(C)C)C2=C1C(=O)[NH]C(=O)[NH]2 | 2421.7 | Standard non polar | 33892256 | | 7-Methyluric acid,1TBDMS,isomer #1 | CN1C(=O)N([Si](C)(C)C(C)(C)C)C2=C1C(=O)[NH]C(=O)[NH]2 | 2891.8 | Standard polar | 33892256 | | 7-Methyluric acid,1TBDMS,isomer #2 | CN1C(=O)[NH]C2=C1C(=O)N([Si](C)(C)C(C)(C)C)C(=O)[NH]2 | 2230.1 | Semi standard non polar | 33892256 | | 7-Methyluric acid,1TBDMS,isomer #2 | CN1C(=O)[NH]C2=C1C(=O)N([Si](C)(C)C(C)(C)C)C(=O)[NH]2 | 2444.3 | Standard non polar | 33892256 | | 7-Methyluric acid,1TBDMS,isomer #2 | CN1C(=O)[NH]C2=C1C(=O)N([Si](C)(C)C(C)(C)C)C(=O)[NH]2 | 2904.1 | Standard polar | 33892256 | | 7-Methyluric acid,1TBDMS,isomer #3 | CN1C(=O)[NH]C2=C1C(=O)[NH]C(=O)N2[Si](C)(C)C(C)(C)C | 2177.2 | Semi standard non polar | 33892256 | | 7-Methyluric acid,1TBDMS,isomer #3 | CN1C(=O)[NH]C2=C1C(=O)[NH]C(=O)N2[Si](C)(C)C(C)(C)C | 2417.9 | Standard non polar | 33892256 | | 7-Methyluric acid,1TBDMS,isomer #3 | CN1C(=O)[NH]C2=C1C(=O)[NH]C(=O)N2[Si](C)(C)C(C)(C)C | 2853.1 | Standard polar | 33892256 | | 7-Methyluric acid,2TBDMS,isomer #1 | CN1C(=O)N([Si](C)(C)C(C)(C)C)C2=C1C(=O)[NH]C(=O)N2[Si](C)(C)C(C)(C)C | 2340.0 | Semi standard non polar | 33892256 | | 7-Methyluric acid,2TBDMS,isomer #1 | CN1C(=O)N([Si](C)(C)C(C)(C)C)C2=C1C(=O)[NH]C(=O)N2[Si](C)(C)C(C)(C)C | 2683.8 | Standard non polar | 33892256 | | 7-Methyluric acid,2TBDMS,isomer #1 | CN1C(=O)N([Si](C)(C)C(C)(C)C)C2=C1C(=O)[NH]C(=O)N2[Si](C)(C)C(C)(C)C | 2586.2 | Standard polar | 33892256 | | 7-Methyluric acid,2TBDMS,isomer #2 | CN1C(=O)N([Si](C)(C)C(C)(C)C)C2=C1C(=O)N([Si](C)(C)C(C)(C)C)C(=O)[NH]2 | 2402.1 | Semi standard non polar | 33892256 | | 7-Methyluric acid,2TBDMS,isomer #2 | CN1C(=O)N([Si](C)(C)C(C)(C)C)C2=C1C(=O)N([Si](C)(C)C(C)(C)C)C(=O)[NH]2 | 2650.6 | Standard non polar | 33892256 | | 7-Methyluric acid,2TBDMS,isomer #2 | CN1C(=O)N([Si](C)(C)C(C)(C)C)C2=C1C(=O)N([Si](C)(C)C(C)(C)C)C(=O)[NH]2 | 2585.0 | Standard polar | 33892256 | | 7-Methyluric acid,2TBDMS,isomer #3 | CN1C(=O)[NH]C2=C1C(=O)N([Si](C)(C)C(C)(C)C)C(=O)N2[Si](C)(C)C(C)(C)C | 2411.1 | Semi standard non polar | 33892256 | | 7-Methyluric acid,2TBDMS,isomer #3 | CN1C(=O)[NH]C2=C1C(=O)N([Si](C)(C)C(C)(C)C)C(=O)N2[Si](C)(C)C(C)(C)C | 2679.0 | Standard non polar | 33892256 | | 7-Methyluric acid,2TBDMS,isomer #3 | CN1C(=O)[NH]C2=C1C(=O)N([Si](C)(C)C(C)(C)C)C(=O)N2[Si](C)(C)C(C)(C)C | 2602.2 | Standard polar | 33892256 | | 7-Methyluric acid,3TBDMS,isomer #1 | CN1C(=O)N([Si](C)(C)C(C)(C)C)C2=C1C(=O)N([Si](C)(C)C(C)(C)C)C(=O)N2[Si](C)(C)C(C)(C)C | 2607.2 | Semi standard non polar | 33892256 | | 7-Methyluric acid,3TBDMS,isomer #1 | CN1C(=O)N([Si](C)(C)C(C)(C)C)C2=C1C(=O)N([Si](C)(C)C(C)(C)C)C(=O)N2[Si](C)(C)C(C)(C)C | 2887.3 | Standard non polar | 33892256 | | 7-Methyluric acid,3TBDMS,isomer #1 | CN1C(=O)N([Si](C)(C)C(C)(C)C)C2=C1C(=O)N([Si](C)(C)C(C)(C)C)C(=O)N2[Si](C)(C)C(C)(C)C | 2590.4 | Standard polar | 33892256 |
|
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - 7-Methyluric acid GC-MS (Non-derivatized) - 70eV, Positive | splash10-03ei-1900000000-48944d897b1adbf7ce06 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 7-Methyluric acid GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental LC-MS/MS | LC-MS/MS Spectrum - 7-Methyluric acid Orbitrap 4V, positive-QTOF | splash10-001i-0900000000-d047affd76adf79850f1 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 7-Methyluric acid Orbitrap 5V, positive-QTOF | splash10-001i-0900000000-586897d99a4bf96f59e9 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 7-Methyluric acid Orbitrap 5V, positive-QTOF | splash10-001i-0900000000-76edc65ffad189950def | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 7-Methyluric acid Orbitrap 6V, positive-QTOF | splash10-001i-0900000000-98bd5bea3d7112e121f7 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 7-Methyluric acid Orbitrap 7V, positive-QTOF | splash10-015c-0900000000-9bc9bace27b6ee9a6492 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 7-Methyluric acid Orbitrap 9V, positive-QTOF | splash10-014i-3900000000-0f68eaedf01ce0b10f9e | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 7-Methyluric acid Orbitrap 11V, positive-QTOF | splash10-014i-9600000000-44337706f27a0c5257a0 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 7-Methyluric acid Orbitrap 14V, positive-QTOF | splash10-014i-9100000000-5daafdc771ce214d9f0c | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 7-Methyluric acid Orbitrap 17V, positive-QTOF | splash10-014i-9000000000-d2d2560858af2ff10256 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 7-Methyluric acid Orbitrap 21V, positive-QTOF | splash10-014i-9000000000-af480b14991f0c34f8c9 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 7-Methyluric acid n/a 12V, positive-QTOF | splash10-014l-0900000000-7ad68dcc2845f0521934 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 7-Methyluric acid n/a 12V, positive-QTOF | splash10-014i-9000000000-f647da344adbdf7bfb1b | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 7-Methyluric acid n/a 12V, positive-QTOF | splash10-03di-0900000000-31bc5353954a5354da02 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 7-Methyluric acid n/a 12V, positive-QTOF | splash10-000i-0900000000-e15d4534ccde7995ef2c | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 7-Methyluric acid n/a 12V, positive-QTOF | splash10-03di-0900000000-8cd35e2b2191f4fb94a1 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 7-Methyluric acid Orbitrap 4V, positive-QTOF | splash10-0006-1900000000-0ea800756f4ab5ea3d25 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 7-Methyluric acid Orbitrap 5V, positive-QTOF | splash10-0006-4900000000-eb83967fc3d3b0d6d8f7 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 7-Methyluric acid Orbitrap 6V, positive-QTOF | splash10-014l-9600000000-f093dd738be634d15c18 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 7-Methyluric acid Orbitrap 8V, positive-QTOF | splash10-014i-9200000000-4325df2e79ca823ab375 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 7-Methyluric acid 10V, Positive-QTOF | splash10-001i-0900000000-dc350a0d473282f926d8 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 7-Methyluric acid 20V, Positive-QTOF | splash10-003u-1900000000-a13ac64bd9ab51761cdd | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 7-Methyluric acid 40V, Positive-QTOF | splash10-000f-9400000000-81688eb86cc318dfd7bb | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 7-Methyluric acid 10V, Negative-QTOF | splash10-001i-0900000000-cf50c6b34fdb182cc93c | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 7-Methyluric acid 20V, Negative-QTOF | splash10-001r-1900000000-42d1e6a7e4e45ebdf17f | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 7-Methyluric acid 40V, Negative-QTOF | splash10-0006-9200000000-7a39d0c29427d4f8cf7f | 2017-09-01 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum |
IR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M-H]-) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+H]+) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+Na]+) | 2023-02-03 | FELIX lab | View Spectrum |
|
|---|