| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected and Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2022-09-22 17:43:42 UTC |
|---|
| HMDB ID | HMDB0000363 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 17a-Hydroxypregnenolone |
|---|
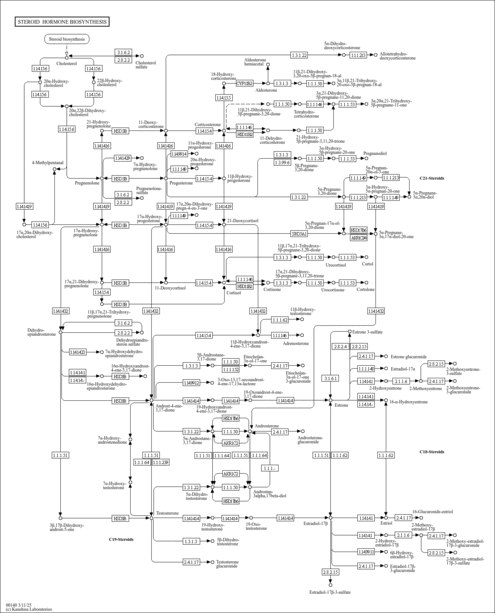
| Description | 17a-Hydroxypregnenolone is a 21-carbon steroid that is converted from pregnenolone by cytochrome P450 17alpha hydroxylase/C17,20 lyase (CYP17, EC 1.14.99.9). 17a-Hydroxypregnenolone is an intermediate in the delta-5 pathway of biosynthesis of gonadal steroid hormones and the adrenal corticosteroids. The first, rate-limiting and hormonally regulated step in the biosynthesis of all steroid hormones is the conversion of cholesterol to pregnenolone. The conversion of cholesterol to pregnenolone is accomplished by the cleavage of the cholesterol side chain, catalyzed by a mitochondrial cytochrome P450 enzyme termed P450scc where scc designates Side Chain Cleavage. All steroid hormones are made from the pregnenolone produced by P450scc; thus, the presence or absence of each of the activities of CYP17 directs this pregnenolone towards its final metabolic pathway. While all cytochrome P450 enzymes can catalyze multiple reactions on a single active site, CYP17 is the only one described to date in which these multiple activities are differentially regulated by a physiologic process. 17a-Hydroxypregnenolone is converted to dehydroepiandrosterone by the 17,20 lyase activity of CYP17. The ratio of the 17,20 lyase to 17 alpha-hydroxylase activity of CYP17 determines the ratio of C21 to C19 steroids produced. This ratio is regulated post-translationally by at least three factors: the abundance of the electron-donating protein P450 oxidoreductase, the presence of cytochrome b5, and the serine phosphorylation of CYP17. (PMID: 12573809 ). |
|---|
| Structure | [H][C@@]12CC[C@](O)(C(C)=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC=C2C[C@@H](O)CC[C@]12C InChI=1S/C21H32O3/c1-13(22)21(24)11-8-18-16-5-4-14-12-15(23)6-9-19(14,2)17(16)7-10-20(18,21)3/h4,15-18,23-24H,5-12H2,1-3H3/t15-,16+,17-,18-,19-,20-,21-/m0/s1 |
|---|
| Synonyms | | Value | Source |
|---|
| (3beta)-3,17-Dihydroxypregn-5-en-20-one | ChEBI | | 17-Hydroxypregnenolone | ChEBI | | 5-Pregnen-3beta,17alpha-diol-20-one | ChEBI | | (3b)-3,17-Dihydroxypregn-5-en-20-one | Generator | | (3Β)-3,17-dihydroxypregn-5-en-20-one | Generator | | 5-Pregnen-3b,17a-diol-20-one | Generator | | 5-Pregnen-3β,17α-diol-20-one | Generator | | 17-Hydroxy-D5-pregnenolone | HMDB | | 17-OH-Pregnenolone | HMDB | | 17a-Hydroxypregnolone | HMDB | | 17alpha-Hydroxypregnanolone | HMDB | | 17alpha-Hydroxypregnenolone | HMDB | | 3b,17-Dihydroxy-5-pregnen-20-one | HMDB | | 3b,17-Dihydroxy-pregn-5-en-20-one | HMDB | | 3b,17a-Dihydroxypregn-5-en-20-one | HMDB | | 17alpha Hydroxypregnenolone | HMDB | | Hydroxypregnenolone | HMDB | | 17 Hydroxypregnenolone | HMDB | | 17 alpha Hydroxypregnenolone | HMDB | | 17-Hydroxypregnenolone, (3alpha)-isomer | HMDB | | 17-Hydroxypregnenolone, (3beta,13alpha)-isomer | HMDB | | 17-alpha-Hydroxypregnenolone | HMDB | | 17 alpha-Hydroxypregnenolone | HMDB | | 17-Hydroxypregnenolone, (3beta,13alpha,17alpha)-isomer | HMDB | | 17-Hydroxypregnenolone, (3beta,17alpha)-isomer | HMDB |
|
|---|
| Chemical Formula | C21H32O3 |
|---|
| Average Molecular Weight | 332.477 |
|---|
| Monoisotopic Molecular Weight | 332.23514489 |
|---|
| IUPAC Name | 1-[(1S,2R,5S,10R,11S,14R,15S)-5,14-dihydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-7-en-14-yl]ethan-1-one |
|---|
| Traditional Name | 1-[(1S,2R,5S,10R,11S,14R,15S)-5,14-dihydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-7-en-14-yl]ethanone |
|---|
| CAS Registry Number | 387-79-1 |
|---|
| SMILES | [H][C@@]12CC[C@](O)(C(C)=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC=C2C[C@@H](O)CC[C@]12C |
|---|
| InChI Identifier | InChI=1S/C21H32O3/c1-13(22)21(24)11-8-18-16-5-4-14-12-15(23)6-9-19(14,2)17(16)7-10-20(18,21)3/h4,15-18,23-24H,5-12H2,1-3H3/t15-,16+,17-,18-,19-,20-,21-/m0/s1 |
|---|
| InChI Key | JERGUCIJOXJXHF-TVWVXWENSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as gluco/mineralocorticoids, progestogins and derivatives. These are steroids with a structure based on a hydroxylated prostane moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Pregnane steroids |
|---|
| Direct Parent | Gluco/mineralocorticoids, progestogins and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Progestogin-skeleton
- 20-oxosteroid
- 3-hydroxy-delta-5-steroid
- 3-hydroxysteroid
- 17-hydroxysteroid
- 3-beta-hydroxysteroid
- 3-beta-hydroxy-delta-5-steroid
- Oxosteroid
- Hydroxysteroid
- Delta-5-steroid
- Alpha-hydroxy ketone
- Tertiary alcohol
- Cyclic alcohol
- Secondary alcohol
- Ketone
- Organic oxygen compound
- Organooxygen compound
- Carbonyl group
- Hydrocarbon derivative
- Alcohol
- Organic oxide
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | 266 - 271 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Experimental Collision Cross Sections |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| 17a-Hydroxypregnenolone,1TMS,isomer #1 | CC(=O)[C@@]1(O[Si](C)(C)C)CC[C@H]2[C@@H]3CC=C4C[C@@H](O)CC[C@]4(C)[C@H]3CC[C@@]21C | 2934.1 | Semi standard non polar | 33892256 | | 17a-Hydroxypregnenolone,1TMS,isomer #2 | CC(=O)[C@@]1(O)CC[C@H]2[C@@H]3CC=C4C[C@@H](O[Si](C)(C)C)CC[C@]4(C)[C@H]3CC[C@@]21C | 2913.3 | Semi standard non polar | 33892256 | | 17a-Hydroxypregnenolone,1TMS,isomer #3 | C=C(O[Si](C)(C)C)[C@@]1(O)CC[C@H]2[C@@H]3CC=C4C[C@@H](O)CC[C@]4(C)[C@H]3CC[C@@]21C | 2827.9 | Semi standard non polar | 33892256 | | 17a-Hydroxypregnenolone,2TMS,isomer #1 | CC(=O)[C@@]1(O[Si](C)(C)C)CC[C@H]2[C@@H]3CC=C4C[C@@H](O[Si](C)(C)C)CC[C@]4(C)[C@H]3CC[C@@]21C | 2961.6 | Semi standard non polar | 33892256 | | 17a-Hydroxypregnenolone,2TMS,isomer #2 | C=C(O[Si](C)(C)C)[C@@]1(O[Si](C)(C)C)CC[C@H]2[C@@H]3CC=C4C[C@@H](O)CC[C@]4(C)[C@H]3CC[C@@]21C | 2958.2 | Semi standard non polar | 33892256 | | 17a-Hydroxypregnenolone,2TMS,isomer #3 | C=C(O[Si](C)(C)C)[C@@]1(O)CC[C@H]2[C@@H]3CC=C4C[C@@H](O[Si](C)(C)C)CC[C@]4(C)[C@H]3CC[C@@]21C | 2855.0 | Semi standard non polar | 33892256 | | 17a-Hydroxypregnenolone,3TMS,isomer #1 | C=C(O[Si](C)(C)C)[C@@]1(O[Si](C)(C)C)CC[C@H]2[C@@H]3CC=C4C[C@@H](O[Si](C)(C)C)CC[C@]4(C)[C@H]3CC[C@@]21C | 2964.5 | Semi standard non polar | 33892256 | | 17a-Hydroxypregnenolone,3TMS,isomer #1 | C=C(O[Si](C)(C)C)[C@@]1(O[Si](C)(C)C)CC[C@H]2[C@@H]3CC=C4C[C@@H](O[Si](C)(C)C)CC[C@]4(C)[C@H]3CC[C@@]21C | 2904.0 | Standard non polar | 33892256 | | 17a-Hydroxypregnenolone,3TMS,isomer #1 | C=C(O[Si](C)(C)C)[C@@]1(O[Si](C)(C)C)CC[C@H]2[C@@H]3CC=C4C[C@@H](O[Si](C)(C)C)CC[C@]4(C)[C@H]3CC[C@@]21C | 3228.3 | Standard polar | 33892256 | | 17a-Hydroxypregnenolone,1TBDMS,isomer #1 | CC(=O)[C@@]1(O[Si](C)(C)C(C)(C)C)CC[C@H]2[C@@H]3CC=C4C[C@@H](O)CC[C@]4(C)[C@H]3CC[C@@]21C | 3183.7 | Semi standard non polar | 33892256 | | 17a-Hydroxypregnenolone,1TBDMS,isomer #2 | CC(=O)[C@@]1(O)CC[C@H]2[C@@H]3CC=C4C[C@@H](O[Si](C)(C)C(C)(C)C)CC[C@]4(C)[C@H]3CC[C@@]21C | 3170.5 | Semi standard non polar | 33892256 | | 17a-Hydroxypregnenolone,1TBDMS,isomer #3 | C=C(O[Si](C)(C)C(C)(C)C)[C@@]1(O)CC[C@H]2[C@@H]3CC=C4C[C@@H](O)CC[C@]4(C)[C@H]3CC[C@@]21C | 3115.1 | Semi standard non polar | 33892256 | | 17a-Hydroxypregnenolone,2TBDMS,isomer #1 | CC(=O)[C@@]1(O[Si](C)(C)C(C)(C)C)CC[C@H]2[C@@H]3CC=C4C[C@@H](O[Si](C)(C)C(C)(C)C)CC[C@]4(C)[C@H]3CC[C@@]21C | 3457.9 | Semi standard non polar | 33892256 | | 17a-Hydroxypregnenolone,2TBDMS,isomer #2 | C=C(O[Si](C)(C)C(C)(C)C)[C@@]1(O[Si](C)(C)C(C)(C)C)CC[C@H]2[C@@H]3CC=C4C[C@@H](O)CC[C@]4(C)[C@H]3CC[C@@]21C | 3503.7 | Semi standard non polar | 33892256 | | 17a-Hydroxypregnenolone,2TBDMS,isomer #3 | C=C(O[Si](C)(C)C(C)(C)C)[C@@]1(O)CC[C@H]2[C@@H]3CC=C4C[C@@H](O[Si](C)(C)C(C)(C)C)CC[C@]4(C)[C@H]3CC[C@@]21C | 3385.0 | Semi standard non polar | 33892256 | | 17a-Hydroxypregnenolone,3TBDMS,isomer #1 | C=C(O[Si](C)(C)C(C)(C)C)[C@@]1(O[Si](C)(C)C(C)(C)C)CC[C@H]2[C@@H]3CC=C4C[C@@H](O[Si](C)(C)C(C)(C)C)CC[C@]4(C)[C@H]3CC[C@@]21C | 3724.7 | Semi standard non polar | 33892256 | | 17a-Hydroxypregnenolone,3TBDMS,isomer #1 | C=C(O[Si](C)(C)C(C)(C)C)[C@@]1(O[Si](C)(C)C(C)(C)C)CC[C@H]2[C@@H]3CC=C4C[C@@H](O[Si](C)(C)C(C)(C)C)CC[C@]4(C)[C@H]3CC[C@@]21C | 3605.8 | Standard non polar | 33892256 | | 17a-Hydroxypregnenolone,3TBDMS,isomer #1 | C=C(O[Si](C)(C)C(C)(C)C)[C@@]1(O[Si](C)(C)C(C)(C)C)CC[C@H]2[C@@H]3CC=C4C[C@@H](O[Si](C)(C)C(C)(C)C)CC[C@]4(C)[C@H]3CC[C@@]21C | 3481.9 | Standard polar | 33892256 |
|
|---|
| Spectra |
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental GC-MS | GC-MS Spectrum - 17a-Hydroxypregnenolone GC-MS (1 MEOX; 2 TMS) | splash10-054x-4900000000-df86d10979d808f20e67 | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - 17a-Hydroxypregnenolone GC-MS (1 MEOX; 2 TMS) | splash10-0a73-3910000000-7a0eea991ac4d2fac97e | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - 17a-Hydroxypregnenolone EI-B (Non-derivatized) | splash10-0f89-2891000000-3605ad7dd57f97382115 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - 17a-Hydroxypregnenolone EI-B (Non-derivatized) | splash10-0ff0-3792000000-974e157d4c25e376b004 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - 17a-Hydroxypregnenolone GC-MS (Non-derivatized) | splash10-054x-4900000000-df86d10979d808f20e67 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - 17a-Hydroxypregnenolone GC-MS (Non-derivatized) | splash10-0a73-3910000000-7a0eea991ac4d2fac97e | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 17a-Hydroxypregnenolone GC-MS (Non-derivatized) - 70eV, Positive | splash10-000f-3295000000-48ed11374901b134a70d | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 17a-Hydroxypregnenolone GC-MS (2 TMS) - 70eV, Positive | splash10-03di-0111900000-e55e71b4d8c63cde89c2 | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 17a-Hydroxypregnenolone GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 17a-Hydroxypregnenolone GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 17a-Hydroxypregnenolone GC-MS (TMS_1_1) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 17a-Hydroxypregnenolone GC-MS (TMS_1_2) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 17a-Hydroxypregnenolone GC-MS (TMS_1_3) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 17a-Hydroxypregnenolone GC-MS (TMS_2_2) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 17a-Hydroxypregnenolone GC-MS (TMS_2_3) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 17a-Hydroxypregnenolone GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 17a-Hydroxypregnenolone GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 17a-Hydroxypregnenolone GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 17a-Hydroxypregnenolone GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 17a-Hydroxypregnenolone GC-MS (TBDMS_2_2) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 17a-Hydroxypregnenolone GC-MS (TBDMS_2_3) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental LC-MS/MS | LC-MS/MS Spectrum - 17a-Hydroxypregnenolone Quattro_QQQ 10V, Positive-QTOF (Annotated) | splash10-001i-0039000000-28c103ca6cdf072557bf | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 17a-Hydroxypregnenolone Quattro_QQQ 25V, Positive-QTOF (Annotated) | splash10-0a4i-0900000000-ae3004bb674d9632cd0c | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 17a-Hydroxypregnenolone Quattro_QQQ 40V, Positive-QTOF (Annotated) | splash10-014i-0900000000-a2f467560b451d5c9b0e | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 17a-Hydroxypregnenolone EI-B (HITACHI M-52) , Positive-QTOF | splash10-0f89-2891000000-82e62584fff36943bcba | 2012-08-31 | HMDB team, MONA | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 17a-Hydroxypregnenolone 10V, Positive-QTOF | splash10-0159-0029000000-131b75950fb7123106d9 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 17a-Hydroxypregnenolone 20V, Positive-QTOF | splash10-015i-0094000000-2a48017855d3965d5626 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 17a-Hydroxypregnenolone 40V, Positive-QTOF | splash10-0g29-2690000000-3d1b2b0525a2f6da149c | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 17a-Hydroxypregnenolone 10V, Negative-QTOF | splash10-001i-0009000000-4c286aa6d88850e66ba9 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 17a-Hydroxypregnenolone 20V, Negative-QTOF | splash10-008i-0098000000-77ce542de402b225ac27 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 17a-Hydroxypregnenolone 40V, Negative-QTOF | splash10-0avr-0092000000-e39f57cc01b46a017728 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 17a-Hydroxypregnenolone 10V, Negative-QTOF | splash10-001i-0009000000-08a5ce4022cbdbf8bfa1 | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 17a-Hydroxypregnenolone 20V, Negative-QTOF | splash10-001i-0029000000-8619dcbabe228085742a | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 17a-Hydroxypregnenolone 40V, Negative-QTOF | splash10-000i-0091000000-4088584830d78ccb6fb2 | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 17a-Hydroxypregnenolone 10V, Positive-QTOF | splash10-00lr-0029000000-275368791dddb24379f9 | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 17a-Hydroxypregnenolone 20V, Positive-QTOF | splash10-014m-0910000000-b08f322c49579d81e79a | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 17a-Hydroxypregnenolone 40V, Positive-QTOF | splash10-0002-1940000000-1f423724847a658c9f85 | 2021-09-24 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Experimental 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, 100%_DMSO, experimental) | 2012-12-05 | Wishart Lab | View Spectrum |
|
|---|
| Biological Properties |
|---|
| Cellular Locations | - Cytoplasm
- Extracellular
- Membrane (predicted from logP)
- Endoplasmic reticulum
|
|---|
| Biospecimen Locations | |
|---|
| Tissue Locations | |
|---|
| Pathways | |
|---|
| Normal Concentrations |
|---|
| |
| Blood | Detected and Quantified | 0.00180-0.00451 uM | Newborn (0-30 days old) | Female | Normal | | details | | Blood | Detected and Quantified | 0.00211-0.0256 uM | Newborn (0-30 days old) | Female | Normal | | details | | Blood | Detected and Quantified | 0.00902 +/- 0.00361 uM | Adult (>18 years old) | Female | Normal | | details | | Blood | Detected and Quantified | 0.000300-0.0240 uM | Infant (0-1 year old) | Not Specified | Normal | | details | | Blood | Detected and Quantified | 0.000782 +/- 0.000993 uM | Children (1-13 years old) | Both | Normal | | details | | Blood | Detected and Quantified | 0.00656 +/- 0.00562 uM | Children (1-13 years old) | Both | Normal | | details | | Blood | Detected and Quantified | 0.003 (0.000 - 0.006) uM | Adult (>18 years old) | Female | Normal | | details | | Blood | Detected and Quantified | 0.006 (0.001-0.012) uM | Adult (>18 years old) | Male | Normal | | details |
|
|---|
| Abnormal Concentrations |
|---|
| |
| Blood | Detected and Quantified | 0.000271 uM | Newborn (0-30 days old) | Female | Adrenal insufficiency, congenital, with 46,XY sex reversal, partial or complete | | details | | Blood | Detected and Quantified | 0.00123 uM | Newborn (0-30 days old) | Female | Adrenal insufficiency, congenital, with 46,XY sex reversal, partial or complete | | details | | Blood | Detected and Quantified | 0.0126 +/- 0.0105 uM | Newborn (0-30 days old) | Not Specified | Adrenal hyperplasia, congenital, due to 3-beta-hydroxysteroid dehydrogenase 2 deficiency | | details | | Blood | Detected and Quantified | 0.00440 +/- 0.00280 uM | Children (1-13 years old) | Not Specified | Adrenal hyperplasia, congenital, due to 3-beta-hydroxysteroid dehydrogenase 2 deficiency | | details | | Blood | Detected and Quantified | 0.159 uM | Adult (>18 years old) | Male | Adrenal hyperplasia, congenital, due to 3-beta-hydroxysteroid dehydrogenase 2 deficiency | | details | | Blood | Detected and Quantified | 0.741 uM | Adolescent (13-18 years old) | Male | Adrenal hyperplasia, congenital, due to 3-beta-hydroxysteroid dehydrogenase 2 deficiency | | details | | Blood | Detected and Quantified | 0.0264-0.103 uM | Children (1-13 years old) | Both | Adrenal hyperplasia, congenital, due to 3-beta-hydroxysteroid dehydrogenase 2 deficiency | | details | | Blood | Detected and Quantified | 0.0843-1.262 uM | Newborn (0-30 days old) | Both | Adrenal hyperplasia, congenital, due to 3-beta-hydroxysteroid dehydrogenase 2 deficiency | | details | | Blood | Detected and Quantified | 0.00230 +/- 0.00200 uM | Children (1-13 years old) | Both | Adrenal hyperplasia, congenital, due to 3-beta-hydroxysteroid dehydrogenase 2 deficiency | | details | | Blood | Detected and Quantified | 0.00300 +/- 0.00250 uM | Adult (>18 years old) | Female | Adrenal hyperplasia, congenital, due to 3-beta-hydroxysteroid dehydrogenase 2 deficiency | | details | | Blood | Detected and Quantified | 0.00380 +/- 0.00210 uM | Adult (>18 years old) | Male | Adrenal hyperplasia, congenital, due to 3-beta-hydroxysteroid dehydrogenase 2 deficiency | | details | | Blood | Detected and Quantified | 0.00896 +/- 0.00770 uM | Infant (0-1 year old) | Not Specified | Adrenal hyperplasia, congenital, due to 3-beta-hydroxysteroid dehydrogenase 2 deficiency | | details | | Blood | Detected and Quantified | 0.0186 uM | Adult (>18 years old) | Female | Adrenal hyperplasia, congenital, due to 3-beta-hydroxysteroid dehydrogenase 2 deficiency | | details | | Blood | Detected and Quantified | 0.0120-0.180 uM | Infant (0-1 year old) | Both | Antley-Bixler syndrome with genital anomalies and disordered steroidogenesis | | details | | Blood | Detected and Quantified | 0.136 +/- 0.129 uM | Children (1-13 years old) | Both | Adrenal hyperplasia, congenital, due to 3-beta-hydroxysteroid dehydrogenase 2 deficiency | | details | | Blood | Detected and Quantified | 0.00439 +/- 0.00896 uM | Children (1-13 years old) | Both | Adrenal hyperplasia, congenital, due to 3-beta-hydroxysteroid dehydrogenase 2 deficiency | | details | | Blood | Detected and Quantified | 0.176 +/- 0.186 uM | Children (1-13 years old) | Both | Adrenal hyperplasia, congenital, due to 3-beta-hydroxysteroid dehydrogenase 2 deficiency | | details | | Blood | Detected and Quantified | 0.0111 +/- 0.0219 uM | Children (1-13 years old) | Both | Adrenal hyperplasia, congenital, due to 3-beta-hydroxysteroid dehydrogenase 2 deficiency | | details | | Urine | Detected but not Quantified | Not Quantified | Not Specified | Not Specified | Cancer patients undergoing total body irradiation | | details |
|
|---|
| Associated Disorders and Diseases |
|---|
| Disease References | | Adrenal hyperplasia, congenital, due to 3-beta-hydroxysteroid dehydrogenase 2 deficiency |
|---|
- Hattori N, Ishihara T, Moridera K, Hino M, Ikekubo K, Kurahachi H: A case of late-onset congenital adrenal hyperplasia due to partial 3 beta-hydroxysteroid dehydrogenase deficiency. Endocr J. 1993 Feb;40(1):107-9. [PubMed:7951484 ]
- Lutfallah C, Wang W, Mason JI, Chang YT, Haider A, Rich B, Castro-Magana M, Copeland KC, David R, Pang S: Newly proposed hormonal criteria via genotypic proof for type II 3beta-hydroxysteroid dehydrogenase deficiency. J Clin Endocrinol Metab. 2002 Jun;87(6):2611-22. doi: 10.1210/jcem.87.6.8615. [PubMed:12050224 ]
- Benkert AR, Young M, Robinson D, Hendrickson C, Lee PA, Strauss KA: Severe Salt-Losing 3beta-Hydroxysteroid Dehydrogenase Deficiency: Treatment and Outcomes of HSD3B2 c.35G>A Homozygotes. J Clin Endocrinol Metab. 2015 Aug;100(8):E1105-15. doi: 10.1210/jc.2015-2098. Epub 2015 Jun 16. [PubMed:26079780 ]
| | Antley-Bixler syndrome with genital anomalies and disordered steroidogenesis |
|---|
- Fukami M, Hasegawa T, Horikawa R, Ohashi T, Nishimura G, Homma K, Ogata T: Cytochrome P450 oxidoreductase deficiency in three patients initially regarded as having 21-hydroxylase deficiency and/or aromatase deficiency: diagnostic value of urine steroid hormone analysis. Pediatr Res. 2006 Feb;59(2):276-80. doi: 10.1203/01.pdr.0000195825.31504.28. [PubMed:16439592 ]
| | Adrenal insufficiency, congenital, with 46,XY sex reversal, partial or complete |
|---|
- Kim CJ, Lin L, Huang N, Quigley CA, AvRuskin TW, Achermann JC, Miller WL: Severe combined adrenal and gonadal deficiency caused by novel mutations in the cholesterol side chain cleavage enzyme, P450scc. J Clin Endocrinol Metab. 2008 Mar;93(3):696-702. doi: 10.1210/jc.2007-2330. Epub 2008 Jan 8. [PubMed:18182448 ]
|
|
|---|
| Associated OMIM IDs | - 201810 (Adrenal hyperplasia, congenital, due to 3-beta-hydroxysteroid dehydrogenase 2 deficiency)
- 201750 (Antley-Bixler syndrome with genital anomalies and disordered steroidogenesis)
- 613743 (Adrenal insufficiency, congenital, with 46,XY sex reversal, partial or complete)
|
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| Phenol Explorer Compound ID | Not Available |
|---|
| FooDB ID | FDB021982 |
|---|
| KNApSAcK ID | Not Available |
|---|
| Chemspider ID | 82579 |
|---|
| KEGG Compound ID | C05138 |
|---|
| BioCyc ID | CPD66-23 |
|---|
| BiGG ID | 45199 |
|---|
| Wikipedia Link | 17-Hydroxypregnenolone |
|---|
| METLIN ID | 5352 |
|---|
| PubChem Compound | 91451 |
|---|
| PDB ID | Not Available |
|---|
| ChEBI ID | 28750 |
|---|
| Food Biomarker Ontology | Not Available |
|---|
| VMH ID | 17AHPRGNLONE |
|---|
| MarkerDB ID | MDB00000153 |
|---|
| Good Scents ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Kaspar, Emanuel; Hiersemann, Wolfgang; Schenck, Martin. Preparation from their esters of steroid alcohols that are sensitive to acids and alkali. (1958), DE 1028572 19580424 CAN 54:74845 AN 1960:74845 CAPLUS |
|---|
| Material Safety Data Sheet (MSDS) | Download (PDF) |
|---|
| General References | - Shimozawa K, Saisho S, Yata J, Kambegawa A: Age-related changes in serum 17-hydroxypregnenolone and 17-hydroxypregnenolone sulfate concentrations in human infancy and childhood. Endocrinol Jpn. 1988 Apr;35(2):189-95. [PubMed:3208699 ]
- Rodriguez-Rigau LJ, Weiss DB, Smith KD, Steinberger E: Suggestion of abnormal testicular steroidogenesis in some oligospermic men. Acta Endocrinol (Copenh). 1978 Feb;87(2):400-12. [PubMed:580138 ]
- Kaufman FR, Costin G, Goebelsmann U, Stanczyk FZ, Zachmann M: Male pseudohermaphroditism due to 17,20-desmolase deficiency. J Clin Endocrinol Metab. 1983 Jul;57(1):32-6. [PubMed:6853680 ]
- Miller WL: Androgen biosynthesis from cholesterol to DHEA. Mol Cell Endocrinol. 2002 Dec 30;198(1-2):7-14. [PubMed:12573809 ]
|
|---|