| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected and Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2021-09-14 15:45:41 UTC |
|---|
| HMDB ID | HMDB0001173 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 5'-Methylthioadenosine |
|---|
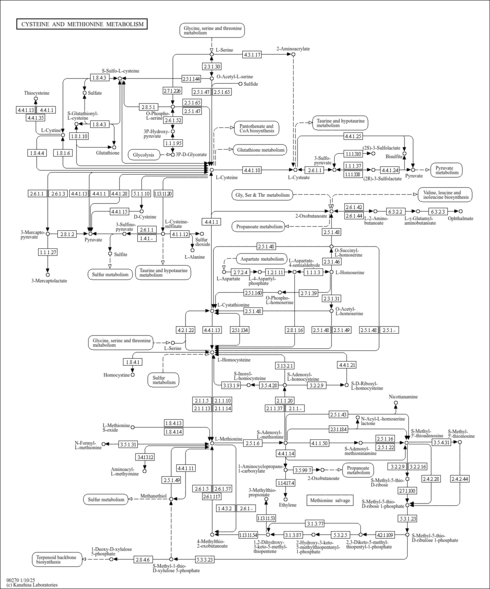
| Description | 5'-Methylthioadenosine, also known as MTA or thiomethyladenosine, belongs to the class of organic compounds known as 5'-deoxy-5'-thionucleosides. These are 5'-deoxyribonucleosides in which the ribose is thio-substituted at the 5'position by a S-alkyl group. 5'-Methylthioadenosine is metabolized solely by MTA-phosphorylase, to yield 5-methylthioribose-1-phosphate and adenine, a crucial step in the methionine and purine salvage pathways, respectively. 5'-Methylthioadenosine exists in all living species, ranging from bacteria to humans. 5'-Methylthioadenosine (MTA) is a naturally occurring sulfur-containing nucleoside present in all mammalian tissues. Within humans, 5'-methylthioadenosine participates in a number of enzymatic reactions. In particular, 5'-methylthioadenosine and spermidine can be biosynthesized from S-adenosylmethioninamine and putrescine through the action of the enzyme spermidine synthase. In addition, 5'-methylthioadenosine can be converted into 5-methylthioribose 1-phosphate and L-methionine; which is catalyzed by the enzyme S-methyl-5'-thioadenosine phosphorylase. It is produced from S-adenosylmethionine mainly through the polyamine biosynthetic pathway, where it behaves as a powerful inhibitory product. For instance, 5'-Methylthioadenosine has been shown to influence the regulation of gene expression, proliferation, differentiation, and apoptosis (PMID:15313459 ). In humans, 5'-methylthioadenosine is involved in the metabolic disorder called hypermethioninemia. Outside of the human body, 5'-Methylthioadenosine has been detected, but not quantified in several different foods, such as soursops, allspices, summer grapes, alaska wild rhubarbs, and breadfruits. Elevated excretion appears in children with severe combined immunodeficiency syndrome (SCID) (PMID:3987052 ). Evidence suggests that 5'-Methylthioadenosine can affect cellular processes in many ways. 5'-Methylthioadenosine can be found in human urine. |
|---|
| Structure | CSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)N1C=NC2=C1N=CN=C2N InChI=1S/C11H15N5O3S/c1-20-2-5-7(17)8(18)11(19-5)16-4-15-6-9(12)13-3-14-10(6)16/h3-5,7-8,11,17-18H,2H2,1H3,(H2,12,13,14)/t5-,7-,8-,11-/m1/s1 |
|---|
| Synonyms | | Value | Source |
|---|
| 5'-Deoxy-5'-(methylthio)adenosine | ChEBI | | 5-Methylthioadenosine | ChEBI | | 9-(5-S-Methyl-5-thio-beta-D-ribofuranosyl)-9H-purin-6-amine | ChEBI | | Methylthioadenosine | ChEBI | | MTA | ChEBI | | S-Methyl-5'-thioadenosine | ChEBI | | Thiomethyladenosine | ChEBI | | 9-(5-S-Methyl-5-thio-b-D-ribofuranosyl)-9H-purin-6-amine | Generator | | 9-(5-S-Methyl-5-thio-β-D-ribofuranosyl)-9H-purin-6-amine | Generator | | 1-(6-Amino-9H-purin-9-yl)-1-deoxy-5-S-methyl-5-thio-beta-D-ribofuranose | HMDB | | 1-(6-Amino-9H-purin-9-yl)-1-deoxy-5-S-methyl-5-thio-beta-delta-ribofuranose | HMDB | | 5'-(Methylthio)-5'-deoxyadenosine | HMDB | | 5'-(Methylthio)adenosine | HMDB | | 5'-S-Methyl-5'-thio-adenosine | HMDB | | 5'-S-Methyl-5'-thioadenosine | HMDB | | S-Methyl-5-thioadenosine | HMDB | | Adenine(5'-deoxy-5'-methylthio)9-beta-D-furanoriboside | HMDB | | 5-MTDA | HMDB | | 5'-Methylthio-5'-deoxyadenosine | HMDB | | 5'-Deoxy-5'-methylthioadenosine | HMDB | | 5'-Methylthioadenosine, methyl-(14)C-labeled | HMDB | | 5'-Methylthioadenosine | ChEBI |
|
|---|
| Chemical Formula | C11H15N5O3S |
|---|
| Average Molecular Weight | 297.334 |
|---|
| Monoisotopic Molecular Weight | 297.089560061 |
|---|
| IUPAC Name | (2R,3R,4S,5S)-2-(6-amino-9H-purin-9-yl)-5-[(methylsulfanyl)methyl]oxolane-3,4-diol |
|---|
| Traditional Name | methylthioadenosine |
|---|
| CAS Registry Number | 2457-80-9 |
|---|
| SMILES | CSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)N1C=NC2=C1N=CN=C2N |
|---|
| InChI Identifier | InChI=1S/C11H15N5O3S/c1-20-2-5-7(17)8(18)11(19-5)16-4-15-6-9(12)13-3-14-10(6)16/h3-5,7-8,11,17-18H,2H2,1H3,(H2,12,13,14)/t5-,7-,8-,11-/m1/s1 |
|---|
| InChI Key | WUUGFSXJNOTRMR-IOSLPCCCSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as 5'-deoxy-5'-thionucleosides. These are 5'-deoxyribonucleosides in which the ribose is thio-substituted at the 5'position by a S-alkyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Nucleosides, nucleotides, and analogues |
|---|
| Class | 5'-deoxyribonucleosides |
|---|
| Sub Class | 5'-deoxy-5'-thionucleosides |
|---|
| Direct Parent | 5'-deoxy-5'-thionucleosides |
|---|
| Alternative Parents | |
|---|
| Substituents | - 5'-deoxy-5'-thionucleoside
- Glycosyl compound
- N-glycosyl compound
- 6-aminopurine
- Pentose monosaccharide
- Imidazopyrimidine
- Purine
- Aminopyrimidine
- Monosaccharide
- N-substituted imidazole
- Pyrimidine
- Imidolactam
- Azole
- Tetrahydrofuran
- Imidazole
- Heteroaromatic compound
- Secondary alcohol
- 1,2-diol
- Organoheterocyclic compound
- Dialkylthioether
- Sulfenyl compound
- Thioether
- Azacycle
- Oxacycle
- Organic oxygen compound
- Alcohol
- Hydrocarbon derivative
- Organopnictogen compound
- Amine
- Primary amine
- Organic nitrogen compound
- Organonitrogen compound
- Organooxygen compound
- Organosulfur compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Not Available | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Experimental Collision Cross Sections |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| 5'-Methylthioadenosine,1TMS,isomer #1 | CSC[C@H]1O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1O | 2746.4 | Semi standard non polar | 33892256 | | 5'-Methylthioadenosine,1TMS,isomer #2 | CSC[C@H]1O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@H](O)[C@@H]1O[Si](C)(C)C | 2744.3 | Semi standard non polar | 33892256 | | 5'-Methylthioadenosine,1TMS,isomer #3 | CSC[C@H]1O[C@@H](N2C=NC3=C(N[Si](C)(C)C)N=CN=C32)[C@H](O)[C@@H]1O | 2775.8 | Semi standard non polar | 33892256 | | 5'-Methylthioadenosine,2TMS,isomer #1 | CSC[C@H]1O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C | 2689.2 | Semi standard non polar | 33892256 | | 5'-Methylthioadenosine,2TMS,isomer #2 | CSC[C@H]1O[C@@H](N2C=NC3=C(N[Si](C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1O | 2743.4 | Semi standard non polar | 33892256 | | 5'-Methylthioadenosine,2TMS,isomer #3 | CSC[C@H]1O[C@@H](N2C=NC3=C(N[Si](C)(C)C)N=CN=C32)[C@H](O)[C@@H]1O[Si](C)(C)C | 2738.7 | Semi standard non polar | 33892256 | | 5'-Methylthioadenosine,2TMS,isomer #4 | CSC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@H](O)[C@@H]1O | 2733.3 | Semi standard non polar | 33892256 | | 5'-Methylthioadenosine,3TMS,isomer #1 | CSC[C@H]1O[C@@H](N2C=NC3=C(N[Si](C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C | 2739.7 | Semi standard non polar | 33892256 | | 5'-Methylthioadenosine,3TMS,isomer #1 | CSC[C@H]1O[C@@H](N2C=NC3=C(N[Si](C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C | 2769.4 | Standard non polar | 33892256 | | 5'-Methylthioadenosine,3TMS,isomer #1 | CSC[C@H]1O[C@@H](N2C=NC3=C(N[Si](C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C | 3998.1 | Standard polar | 33892256 | | 5'-Methylthioadenosine,3TMS,isomer #2 | CSC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1O | 2744.4 | Semi standard non polar | 33892256 | | 5'-Methylthioadenosine,3TMS,isomer #2 | CSC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1O | 2825.2 | Standard non polar | 33892256 | | 5'-Methylthioadenosine,3TMS,isomer #2 | CSC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1O | 3755.0 | Standard polar | 33892256 | | 5'-Methylthioadenosine,3TMS,isomer #3 | CSC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@H](O)[C@@H]1O[Si](C)(C)C | 2733.0 | Semi standard non polar | 33892256 | | 5'-Methylthioadenosine,3TMS,isomer #3 | CSC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@H](O)[C@@H]1O[Si](C)(C)C | 2817.3 | Standard non polar | 33892256 | | 5'-Methylthioadenosine,3TMS,isomer #3 | CSC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@H](O)[C@@H]1O[Si](C)(C)C | 3702.1 | Standard polar | 33892256 | | 5'-Methylthioadenosine,4TMS,isomer #1 | CSC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C | 2759.5 | Semi standard non polar | 33892256 | | 5'-Methylthioadenosine,4TMS,isomer #1 | CSC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C | 2839.7 | Standard non polar | 33892256 | | 5'-Methylthioadenosine,4TMS,isomer #1 | CSC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C)[Si](C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C)[C@@H]1O[Si](C)(C)C | 3414.5 | Standard polar | 33892256 | | 5'-Methylthioadenosine,1TBDMS,isomer #1 | CSC[C@H]1O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O | 2964.2 | Semi standard non polar | 33892256 | | 5'-Methylthioadenosine,1TBDMS,isomer #2 | CSC[C@H]1O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@H](O)[C@@H]1O[Si](C)(C)C(C)(C)C | 2959.6 | Semi standard non polar | 33892256 | | 5'-Methylthioadenosine,1TBDMS,isomer #3 | CSC[C@H]1O[C@@H](N2C=NC3=C(N[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O)[C@@H]1O | 2958.8 | Semi standard non polar | 33892256 | | 5'-Methylthioadenosine,2TBDMS,isomer #1 | CSC[C@H]1O[C@@H](N2C=NC3=C(N)N=CN=C32)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C | 3099.4 | Semi standard non polar | 33892256 | | 5'-Methylthioadenosine,2TBDMS,isomer #2 | CSC[C@H]1O[C@@H](N2C=NC3=C(N[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O | 3098.7 | Semi standard non polar | 33892256 | | 5'-Methylthioadenosine,2TBDMS,isomer #3 | CSC[C@H]1O[C@@H](N2C=NC3=C(N[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O)[C@@H]1O[Si](C)(C)C(C)(C)C | 3088.3 | Semi standard non polar | 33892256 | | 5'-Methylthioadenosine,2TBDMS,isomer #4 | CSC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O)[C@@H]1O | 3120.6 | Semi standard non polar | 33892256 | | 5'-Methylthioadenosine,3TBDMS,isomer #1 | CSC[C@H]1O[C@@H](N2C=NC3=C(N[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C | 3298.2 | Semi standard non polar | 33892256 | | 5'-Methylthioadenosine,3TBDMS,isomer #1 | CSC[C@H]1O[C@@H](N2C=NC3=C(N[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C | 3388.9 | Standard non polar | 33892256 | | 5'-Methylthioadenosine,3TBDMS,isomer #1 | CSC[C@H]1O[C@@H](N2C=NC3=C(N[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C | 4073.9 | Standard polar | 33892256 | | 5'-Methylthioadenosine,3TBDMS,isomer #2 | CSC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O | 3265.8 | Semi standard non polar | 33892256 | | 5'-Methylthioadenosine,3TBDMS,isomer #2 | CSC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O | 3445.2 | Standard non polar | 33892256 | | 5'-Methylthioadenosine,3TBDMS,isomer #2 | CSC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O | 3846.1 | Standard polar | 33892256 | | 5'-Methylthioadenosine,3TBDMS,isomer #3 | CSC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O)[C@@H]1O[Si](C)(C)C(C)(C)C | 3254.8 | Semi standard non polar | 33892256 | | 5'-Methylthioadenosine,3TBDMS,isomer #3 | CSC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O)[C@@H]1O[Si](C)(C)C(C)(C)C | 3441.8 | Standard non polar | 33892256 | | 5'-Methylthioadenosine,3TBDMS,isomer #3 | CSC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O)[C@@H]1O[Si](C)(C)C(C)(C)C | 3805.5 | Standard polar | 33892256 | | 5'-Methylthioadenosine,4TBDMS,isomer #1 | CSC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C | 3460.7 | Semi standard non polar | 33892256 | | 5'-Methylthioadenosine,4TBDMS,isomer #1 | CSC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C | 3592.9 | Standard non polar | 33892256 | | 5'-Methylthioadenosine,4TBDMS,isomer #1 | CSC[C@H]1O[C@@H](N2C=NC3=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N=CN=C32)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H]1O[Si](C)(C)C(C)(C)C | 3688.6 | Standard polar | 33892256 |
|
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental GC-MS | GC-MS Spectrum - 5'-Methylthioadenosine GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (Non-derivatized) | splash10-000i-0940000000-16b1106966ac98c7a107 | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - 5'-Methylthioadenosine GC-EI-TOF (Non-derivatized) | splash10-000i-0940000000-16b1106966ac98c7a107 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - 5'-Methylthioadenosine GC-EI-TOF (Non-derivatized) | splash10-000i-0940000000-30fc68e7b5ff7576069b | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - 5'-Methylthioadenosine GC-EI-TOF (Non-derivatized) | splash10-01ti-0900000000-312bd8eec3c461e919f9 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 5'-Methylthioadenosine GC-MS (Non-derivatized) - 70eV, Positive | splash10-056r-9530000000-5fbf6b1a856ad3827980 | 2017-09-01 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 5'-Methylthioadenosine GC-MS (2 TMS) - 70eV, Positive | splash10-0h70-9636300000-67e425eba97fe420c464 | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 5'-Methylthioadenosine GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - 5'-Methylthioadenosine GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental LC-MS/MS | LC-MS/MS Spectrum - 5'-Methylthioadenosine LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Negative-QTOF | splash10-001i-0910000000-56784be58ef9065f21f6 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 5'-Methylthioadenosine LC-ESI-QTOF , negative-QTOF | splash10-001i-0910000000-56784be58ef9065f21f6 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 5'-Methylthioadenosine 35V, Negative-QTOF | splash10-001i-0900000000-5f5ce891d3b31bea8dff | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 5'-Methylthioadenosine 35V, Negative-QTOF | splash10-001i-0900000000-d819212647c26720bdfc | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 5'-Methylthioadenosine 10V, Negative-QTOF | splash10-001i-0900000000-8ac1758f0ac9c7d7a1c8 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 5'-Methylthioadenosine 20V, Negative-QTOF | splash10-001i-0900000000-d152916d09f6a5e55194 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 5'-Methylthioadenosine 40V, Negative-QTOF | splash10-001i-0900000000-ffdaf4c3fd84e8313a82 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 5'-Methylthioadenosine Quattro_QQQ 10V, Positive-QTOF (Annotated) | splash10-000j-0940000000-55655e7a0a6c8acba0f0 | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 5'-Methylthioadenosine Quattro_QQQ 25V, Positive-QTOF (Annotated) | splash10-000i-1900000000-f37865ca2029a623ace7 | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 5'-Methylthioadenosine Quattro_QQQ 40V, Positive-QTOF (Annotated) | splash10-000i-2900000000-ae0e3df9bf25c100940d | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 5'-Methylthioadenosine LC-ESI-QQ (API3000, Applied Biosystems) 10V, Positive-QTOF | splash10-0002-0090000000-72139812c5b2ede077ed | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 5'-Methylthioadenosine LC-ESI-QQ (API3000, Applied Biosystems) 20V, Positive-QTOF | splash10-000i-0910000000-0c3d173f36272e3271eb | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 5'-Methylthioadenosine LC-ESI-QQ (API3000, Applied Biosystems) 30V, Positive-QTOF | splash10-000i-0900000000-f3443ca133811f52c5d7 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 5'-Methylthioadenosine LC-ESI-QQ (API3000, Applied Biosystems) 40V, Positive-QTOF | splash10-000i-1900000000-49cc299525d7fca27818 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 5'-Methylthioadenosine LC-ESI-QQ (API3000, Applied Biosystems) 50V, Positive-QTOF | splash10-000i-3900000000-39bebdcaa7ce2695465b | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 5'-Methylthioadenosine LC-ESI-IT (LC/MSD Trap XCT, Agilent Technologies) , Positive-QTOF | splash10-000i-0900000000-76b935f4f0a78c15ca0e | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 5'-Methylthioadenosine LC-ESI-IT (LC/MSD Trap XCT, Agilent Technologies) , Positive-QTOF | splash10-0002-4900000000-d8c0df43305b934d71dc | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 5'-Methylthioadenosine LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Positive-QTOF | splash10-000i-0900000000-d3bfbeb6fb95e3768ef6 | 2012-08-31 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 5'-Methylthioadenosine LC-ESI-QQ , positive-QTOF | splash10-0002-0090000000-72139812c5b2ede077ed | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 5'-Methylthioadenosine LC-ESI-QQ , positive-QTOF | splash10-000i-0910000000-0c3d173f36272e3271eb | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 5'-Methylthioadenosine LC-ESI-QQ , positive-QTOF | splash10-000i-0900000000-f3443ca133811f52c5d7 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 5'-Methylthioadenosine LC-ESI-QQ , positive-QTOF | splash10-000i-1900000000-6ec86a11bf8ad7331bd2 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 5'-Methylthioadenosine LC-ESI-QQ , positive-QTOF | splash10-000i-3900000000-39bebdcaa7ce2695465b | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 5'-Methylthioadenosine LC-ESI-IT , positive-QTOF | splash10-000i-0900000000-b9006ef8eecdaefc89a6 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - 5'-Methylthioadenosine LC-ESI-ITFT , positive-QTOF | splash10-000i-0900000000-949c013f4e9edec10cb9 | 2017-09-14 | HMDB team, MONA | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Experimental 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, experimental) | 2012-12-04 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-24 | Wishart Lab | View Spectrum | | Experimental 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, 100%_DMSO, experimental) | 2012-12-05 | Wishart Lab | View Spectrum |
|
|---|
| General References | - Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM: Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009 Feb 12;457(7231):910-4. doi: 10.1038/nature07762. [PubMed:19212411 ]
- Liebich HM, Di Stefano C, Wixforth A, Schmid HR: Quantitation of urinary nucleosides by high-performance liquid chromatography. J Chromatogr A. 1997 Feb 28;763(1-2):193-7. [PubMed:9129323 ]
- Avila MA, Garcia-Trevijano ER, Lu SC, Corrales FJ, Mato JM: Methylthioadenosine. Int J Biochem Cell Biol. 2004 Nov;36(11):2125-30. [PubMed:15313459 ]
- Gerasimovskaya EV, Ahmad S, White CW, Jones PL, Carpenter TC, Stenmark KR: Extracellular ATP is an autocrine/paracrine regulator of hypoxia-induced adventitial fibroblast growth. Signaling through extracellular signal-regulated kinase-1/2 and the Egr-1 transcription factor. J Biol Chem. 2002 Nov 22;277(47):44638-50. Epub 2002 Sep 18. [PubMed:12244041 ]
- Mills GC, Mills JS: Urinary excretion of methylthioadenosine in immunodeficient children. Clin Chim Acta. 1985 Mar 30;147(1):15-23. [PubMed:3987052 ]
- Contreres JO, Dupuy E, Job B, Habib A, Bryckaert M, Rosa JP, Simoneau G, Herbert JM, Savi P, Levy-Toledano S: Effect of clopidogrel administration to healthy volunteers on platelet phosphorylation events triggered by ADP. Br J Haematol. 2003 Feb;120(4):633-42. [PubMed:12588350 ]
- Mills GC, Schmalstieg FC, Goldblum RM: Urinary excretion of modified purines and nucleosides in immunodeficient children. Biochem Med. 1985 Aug;34(1):37-51. [PubMed:4052062 ]
- Elshenawy S, Pinney SE, Stuart T, Doulias PT, Zura G, Parry S, Elovitz MA, Bennett MJ, Bansal A, Strauss JF 3rd, Ischiropoulos H, Simmons RA: The Metabolomic Signature of the Placenta in Spontaneous Preterm Birth. Int J Mol Sci. 2020 Feb 4;21(3). pii: ijms21031043. doi: 10.3390/ijms21031043. [PubMed:32033212 ]
|
|---|