| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Expected but not Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2020-06-30 18:40:48 UTC |
|---|
| HMDB ID | HMDB0001285 |
|---|
| Secondary Accession Numbers | - HMDB0001560
- HMDB0002239
- HMDB0006506
- HMDB01285
- HMDB01560
- HMDB02239
- HMDB06506
|
|---|
| Metabolite Identification |
|---|
| Common Name | Geranyl-PP |
|---|
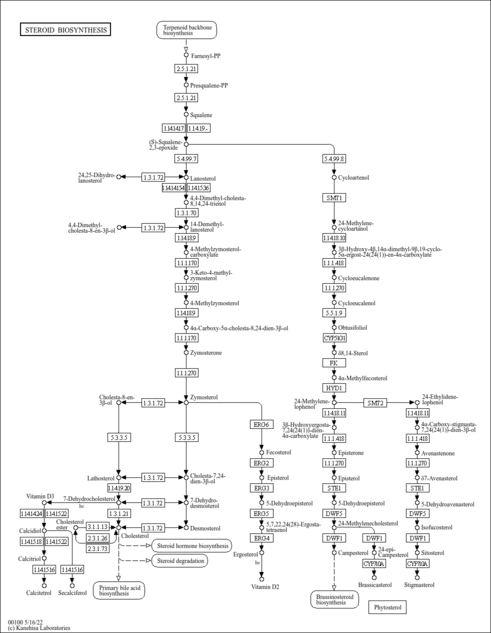
| Description | Geranyl diphosphate is the precursor of monoterpenes, a large family of natural occurring C10 compounds predominately found in plants and animals. Geranyl diphosphate is regarded as a key intermediate in the steroid, isoprene and terpene biosynthesis pathways and is used by organisms in the biosynthesis of farnesyl pyrophosphate, geranylgeranyl pyrophosphate, cholesterol, terpenes and terpenoids. (wikipedia). In humans, geranyl diphosphate synthase (GPPS) catalyzes the condensation of dimethylallyl diphosphate (DMAPP) and isopentenyl diphosphate (IPP) to form geranyl diphosphate. Animals produce IPP through the mevalonate (MVA) pathway. Isoprenoid compounds have been implicated in several human disease states including coronary heart disease, blindness, infectious hepatitis and cancer. Geranyl pyrophosphate is an intermediate in the HMG-CoA reductase pathway used by organisms in the biosynthesis of terpenes and terpenoids. -- Wikipedia . |
|---|
| Structure | CC(C)=CCC\C(C)=C\COP(O)(=O)OP(O)(O)=O InChI=1S/C10H20O7P2/c1-9(2)5-4-6-10(3)7-8-16-19(14,15)17-18(11,12)13/h5,7H,4,6,8H2,1-3H3,(H,14,15)(H2,11,12,13)/b10-7+ |
|---|
| Synonyms | | Value | Source |
|---|
| Geranyl pyrophosphate | ChEBI | | Polyisopentenylpyrophosphate | Kegg | | Polyisopentenyldiphosphate | Kegg | | trans-Polyisopentenyldiphosphate | Kegg | | Polyprenyl diphosphate | Kegg | | Geranyl pyrophosphoric acid | Generator | | Polyisopentenylpyrophosphoric acid | Generator | | Polyisopentenyldiphosphoric acid | Generator | | trans-Polyisopentenyldiphosphoric acid | Generator | | Polyprenyl diphosphoric acid | Generator | | (2E)-3,7-Dimethylocta-2,6-dien-1-yl trihydrogen diphosphate | HMDB | | Geranyl diphosphate | HMDB | | Geranyl-diphosphate | HMDB | | Geranyl-pyrophosphate | HMDB | | Monoterpenyl diphosphate | HMDB | | Neryl diphosphate | HMDB | | trans-Geranyl pyrophosphate | HMDB | | P-[(2E)-3,7-Dimethyl-2,6-octadien-1-yl]diphosphoric acid | HMDB | | trans-geranyl-PP | HMDB |
|
|---|
| Chemical Formula | C10H20O7P2 |
|---|
| Average Molecular Weight | 314.2091 |
|---|
| Monoisotopic Molecular Weight | 314.068426018 |
|---|
| IUPAC Name | [({[(2E)-3,7-dimethylocta-2,6-dien-1-yl]oxy}(hydroxy)phosphoryl)oxy]phosphonic acid |
|---|
| Traditional Name | geranyl diphosphate |
|---|
| CAS Registry Number | 763-10-0 |
|---|
| SMILES | CC(C)=CCC\C(C)=C\COP(O)(=O)OP(O)(O)=O |
|---|
| InChI Identifier | InChI=1S/C10H20O7P2/c1-9(2)5-4-6-10(3)7-8-16-19(14,15)17-18(11,12)13/h5,7H,4,6,8H2,1-3H3,(H,14,15)(H2,11,12,13)/b10-7+ |
|---|
| InChI Key | GVVPGTZRZFNKDS-JXMROGBWSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as isoprenoid phosphates. These are prenol lipids containing a phosphate group linked to an isoprene (2-methylbuta-1,3-diene) unit. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Isoprenoid phosphates |
|---|
| Direct Parent | Isoprenoid phosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Organic pyrophosphate
- Monoterpenoid
- Isoprenoid phosphate
- Acyclic monoterpenoid
- Monoalkyl phosphate
- Alkyl phosphate
- Phosphoric acid ester
- Organic phosphoric acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Experimental Collision Cross Sections |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Retention Times Underivatized| Chromatographic Method | Retention Time | Reference |
|---|
| Measured using a Waters Acquity ultraperformance liquid chromatography (UPLC) ethylene-bridged hybrid (BEH) C18 column (100 mm × 2.1 mm; 1.7 μmparticle diameter). Predicted by Afia on May 17, 2022. Predicted by Afia on May 17, 2022. | 6.44 minutes | 32390414 | | Predicted by Siyang on May 30, 2022 | 10.395 minutes | 33406817 | | Predicted by Siyang using ReTip algorithm on June 8, 2022 | 2.99 minutes | 32390414 | | AjsUoB = Accucore 150 Amide HILIC with 10mM Ammonium Formate, 0.1% Formic Acid | 358.9 seconds | 40023050 | | Fem_Long = Waters ACQUITY UPLC HSS T3 C18 with Water:MeOH and 0.1% Formic Acid | 1378.7 seconds | 40023050 | | Fem_Lipids = Ascentis Express C18 with (60:40 water:ACN):(90:10 IPA:ACN) and 10mM NH4COOH + 0.1% Formic Acid | 218.7 seconds | 40023050 | | Life_Old = Waters ACQUITY UPLC BEH C18 with Water:(20:80 acetone:ACN) and 0.1% Formic Acid | 97.2 seconds | 40023050 | | Life_New = RP Waters ACQUITY UPLC HSS T3 C18 with Water:(30:70 MeOH:ACN) and 0.1% Formic Acid | 166.2 seconds | 40023050 | | RIKEN = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 61.8 seconds | 40023050 | | Eawag_XBridgeC18 = XBridge C18 3.5u 2.1x50 mm with Water:MeOH and 0.1% Formic Acid | 345.8 seconds | 40023050 | | BfG_NTS_RP1 =Agilent Zorbax Eclipse Plus C18 (2.1 mm x 150 mm, 3.5 um) with Water:ACN and 0.1% Formic Acid | 453.7 seconds | 40023050 | | HILIC_BDD_2 = Merck SeQuant ZIC-HILIC with ACN(0.1% formic acid):water(16 mM ammonium formate) | 129.7 seconds | 40023050 | | UniToyama_Atlantis = RP Waters Atlantis T3 (2.1 x 150 mm, 5 um) with ACN:Water and 0.1% Formic Acid | 706.9 seconds | 40023050 | | BDD_C18 = Hypersil Gold 1.9µm C18 with Water:ACN and 0.1% Formic Acid | 408.9 seconds | 40023050 | | UFZ_Phenomenex = Kinetex Core-Shell C18 2.6 um, 3.0 x 100 mm, Phenomenex with Water:MeOH and 0.1% Formic Acid | 547.2 seconds | 40023050 | | SNU_RIKEN_POS = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 252.0 seconds | 40023050 | | RPMMFDA = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 218.6 seconds | 40023050 | | MTBLS87 = Merck SeQuant ZIC-pHILIC column with ACN:Water and :ammonium carbonate | 560.5 seconds | 40023050 | | KI_GIAR_zic_HILIC_pH2_7 = Merck SeQuant ZIC-HILIC with ACN:Water and 0.1% FA | 243.5 seconds | 40023050 | | Meister zic-pHILIC pH9.3 = Merck SeQuant ZIC-pHILIC column with ACN:Water 5mM NH4Ac pH9.3 and 5mM ammonium acetate in water | 172.0 seconds | 40023050 |
Predicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| Geranyl-PP,1TMS,isomer #1 | CC(C)=CCC/C(C)=C/COP(=O)(O[Si](C)(C)C)OP(=O)(O)O | 2299.9 | Semi standard non polar | 33892256 | | Geranyl-PP,1TMS,isomer #1 | CC(C)=CCC/C(C)=C/COP(=O)(O[Si](C)(C)C)OP(=O)(O)O | 2062.8 | Standard non polar | 33892256 | | Geranyl-PP,1TMS,isomer #1 | CC(C)=CCC/C(C)=C/COP(=O)(O[Si](C)(C)C)OP(=O)(O)O | 3125.1 | Standard polar | 33892256 | | Geranyl-PP,1TMS,isomer #2 | CC(C)=CCC/C(C)=C/COP(=O)(O)OP(=O)(O)O[Si](C)(C)C | 2283.6 | Semi standard non polar | 33892256 | | Geranyl-PP,1TMS,isomer #2 | CC(C)=CCC/C(C)=C/COP(=O)(O)OP(=O)(O)O[Si](C)(C)C | 2064.8 | Standard non polar | 33892256 | | Geranyl-PP,1TMS,isomer #2 | CC(C)=CCC/C(C)=C/COP(=O)(O)OP(=O)(O)O[Si](C)(C)C | 3162.0 | Standard polar | 33892256 | | Geranyl-PP,2TMS,isomer #1 | CC(C)=CCC/C(C)=C/COP(=O)(O[Si](C)(C)C)OP(=O)(O)O[Si](C)(C)C | 2320.5 | Semi standard non polar | 33892256 | | Geranyl-PP,2TMS,isomer #1 | CC(C)=CCC/C(C)=C/COP(=O)(O[Si](C)(C)C)OP(=O)(O)O[Si](C)(C)C | 2116.6 | Standard non polar | 33892256 | | Geranyl-PP,2TMS,isomer #1 | CC(C)=CCC/C(C)=C/COP(=O)(O[Si](C)(C)C)OP(=O)(O)O[Si](C)(C)C | 2807.6 | Standard polar | 33892256 | | Geranyl-PP,2TMS,isomer #2 | CC(C)=CCC/C(C)=C/COP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 2323.7 | Semi standard non polar | 33892256 | | Geranyl-PP,2TMS,isomer #2 | CC(C)=CCC/C(C)=C/COP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 2114.0 | Standard non polar | 33892256 | | Geranyl-PP,2TMS,isomer #2 | CC(C)=CCC/C(C)=C/COP(=O)(O)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 2815.4 | Standard polar | 33892256 | | Geranyl-PP,3TMS,isomer #1 | CC(C)=CCC/C(C)=C/COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 2342.7 | Semi standard non polar | 33892256 | | Geranyl-PP,3TMS,isomer #1 | CC(C)=CCC/C(C)=C/COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 2174.2 | Standard non polar | 33892256 | | Geranyl-PP,3TMS,isomer #1 | CC(C)=CCC/C(C)=C/COP(=O)(O[Si](C)(C)C)OP(=O)(O[Si](C)(C)C)O[Si](C)(C)C | 2495.2 | Standard polar | 33892256 | | Geranyl-PP,1TBDMS,isomer #1 | CC(C)=CCC/C(C)=C/COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O | 2531.3 | Semi standard non polar | 33892256 | | Geranyl-PP,1TBDMS,isomer #1 | CC(C)=CCC/C(C)=C/COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O | 2234.5 | Standard non polar | 33892256 | | Geranyl-PP,1TBDMS,isomer #1 | CC(C)=CCC/C(C)=C/COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O | 3265.1 | Standard polar | 33892256 | | Geranyl-PP,1TBDMS,isomer #2 | CC(C)=CCC/C(C)=C/COP(=O)(O)OP(=O)(O)O[Si](C)(C)C(C)(C)C | 2516.0 | Semi standard non polar | 33892256 | | Geranyl-PP,1TBDMS,isomer #2 | CC(C)=CCC/C(C)=C/COP(=O)(O)OP(=O)(O)O[Si](C)(C)C(C)(C)C | 2248.2 | Standard non polar | 33892256 | | Geranyl-PP,1TBDMS,isomer #2 | CC(C)=CCC/C(C)=C/COP(=O)(O)OP(=O)(O)O[Si](C)(C)C(C)(C)C | 3300.4 | Standard polar | 33892256 | | Geranyl-PP,2TBDMS,isomer #1 | CC(C)=CCC/C(C)=C/COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O[Si](C)(C)C(C)(C)C | 2732.1 | Semi standard non polar | 33892256 | | Geranyl-PP,2TBDMS,isomer #1 | CC(C)=CCC/C(C)=C/COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O[Si](C)(C)C(C)(C)C | 2447.5 | Standard non polar | 33892256 | | Geranyl-PP,2TBDMS,isomer #1 | CC(C)=CCC/C(C)=C/COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O)O[Si](C)(C)C(C)(C)C | 2980.3 | Standard polar | 33892256 | | Geranyl-PP,2TBDMS,isomer #2 | CC(C)=CCC/C(C)=C/COP(=O)(O)OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 2739.2 | Semi standard non polar | 33892256 | | Geranyl-PP,2TBDMS,isomer #2 | CC(C)=CCC/C(C)=C/COP(=O)(O)OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 2408.5 | Standard non polar | 33892256 | | Geranyl-PP,2TBDMS,isomer #2 | CC(C)=CCC/C(C)=C/COP(=O)(O)OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 3000.0 | Standard polar | 33892256 | | Geranyl-PP,3TBDMS,isomer #1 | CC(C)=CCC/C(C)=C/COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 2924.4 | Semi standard non polar | 33892256 | | Geranyl-PP,3TBDMS,isomer #1 | CC(C)=CCC/C(C)=C/COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 2613.0 | Standard non polar | 33892256 | | Geranyl-PP,3TBDMS,isomer #1 | CC(C)=CCC/C(C)=C/COP(=O)(O[Si](C)(C)C(C)(C)C)OP(=O)(O[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 2757.9 | Standard polar | 33892256 |
|
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - Geranyl-PP GC-MS (Non-derivatized) - 70eV, Positive | splash10-004j-9640000000-9ce32c835bdcfa97fa15 | 2016-09-22 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Geranyl-PP GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Geranyl-PP GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental LC-MS/MS | LC-MS/MS Spectrum - Geranyl-PP Orbitrap 6V, negative-QTOF | splash10-0002-0090000000-b5ca864b0ba8818ad627 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Geranyl-PP Orbitrap 8V, negative-QTOF | splash10-0002-0090000000-c7f7c5c529a56d532304 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Geranyl-PP Orbitrap 11V, negative-QTOF | splash10-0002-3390000000-010bee66d1e4bd685e95 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Geranyl-PP Orbitrap 14V, negative-QTOF | splash10-004i-9440000000-13e525f66230aae586a2 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Geranyl-PP Orbitrap 17V, negative-QTOF | splash10-004i-9200000000-a9da8f03edb73eec50bf | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Geranyl-PP Orbitrap 20V, negative-QTOF | splash10-004i-9100000000-fe3060700600ee72f8dd | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Geranyl-PP Orbitrap 23V, negative-QTOF | splash10-004i-9000000000-15de26c097afd86b7007 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Geranyl-PP Orbitrap 27V, negative-QTOF | splash10-004i-9000000000-bcc45bf0bf3bbb46af13 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Geranyl-PP Orbitrap 33V, negative-QTOF | splash10-004i-9000000000-b8b56dc9ea06c2ce52b5 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Geranyl-PP n/a 21V, negative-QTOF | splash10-0a4i-0900000000-f2685d197409ed952f02 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Geranyl-PP n/a 21V, negative-QTOF | splash10-004i-9000000000-5ef060d5ec4b80c35c1a | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Geranyl-PP n/a 21V, negative-QTOF | splash10-0a4i-0900000000-60b9e910670cbc6ebe0c | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Geranyl-PP n/a 21V, negative-QTOF | splash10-0bt9-0390000000-6532032bbb594aa54d9c | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Geranyl-PP Orbitrap 5V, negative-QTOF | splash10-03di-0009000000-3bcab8c3688c96a4cec5 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Geranyl-PP Orbitrap 7V, negative-QTOF | splash10-03di-2009000000-42fa98db20ae4d6f687e | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Geranyl-PP Orbitrap 9V, negative-QTOF | splash10-01t9-9005000000-44618200345aed481b48 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Geranyl-PP Orbitrap 12V, negative-QTOF | splash10-004i-9000000000-befed7a961a777eb5f66 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Geranyl-PP Orbitrap 15V, negative-QTOF | splash10-004i-9000000000-8afaaf750b51acbd1a01 | 2020-07-22 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Geranyl-PP Orbitrap 18V, negative-QTOF | splash10-004i-9000000000-b5b3b346fecad6ae473d | 2020-07-22 | HMDB team, MONA | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Geranyl-PP 10V, Positive-QTOF | splash10-00kr-1953000000-e5ee3340554c9ecac23e | 2015-09-14 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Geranyl-PP 20V, Positive-QTOF | splash10-000i-5910000000-82bcd2195a53b847981c | 2015-09-14 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Geranyl-PP 40V, Positive-QTOF | splash10-0gc0-9400000000-f87d391f1b3c01743515 | 2015-09-14 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Geranyl-PP 10V, Negative-QTOF | splash10-03di-0509000000-43083a162145718cd977 | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Geranyl-PP 20V, Negative-QTOF | splash10-004i-9500000000-053b6323e620a16328a8 | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Geranyl-PP 40V, Negative-QTOF | splash10-004i-9000000000-4faed4c921d605619caf | 2015-09-15 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, H2O, predicted) | 2022-08-20 | Wishart Lab | View Spectrum |
|
|---|
| General References | - Kavanagh KL, Guo K, Dunford JE, Wu X, Knapp S, Ebetino FH, Rogers MJ, Russell RG, Oppermann U: The molecular mechanism of nitrogen-containing bisphosphonates as antiosteoporosis drugs. Proc Natl Acad Sci U S A. 2006 May 16;103(20):7829-34. Epub 2006 May 9. [PubMed:16684881 ]
- Micali E, Chehade KA, Isaacs RJ, Andres DA, Spielmann HP: Protein farnesyltransferase isoprenoid substrate discrimination is dependent on isoprene double bonds and branched methyl groups. Biochemistry. 2001 Oct 16;40(41):12254-65. [PubMed:11591144 ]
- Holstein SA, Hohl RJ: Isoprenoids: remarkable diversity of form and function. Lipids. 2004 Apr;39(4):293-309. [PubMed:15357017 ]
- Gan X, Kaplan R, Menke JG, MacNaul K, Chen Y, Sparrow CP, Zhou G, Wright SD, Cai TQ: Dual mechanisms of ABCA1 regulation by geranylgeranyl pyrophosphate. J Biol Chem. 2001 Dec 28;276(52):48702-8. Epub 2001 Oct 18. [PubMed:11641412 ]
- Sagami H, Ogura K: [A new development in isoprenoid biochemistry brought by the discovery of prenylated proteins]. Seikagaku. 1994 Dec;66(12):1488-501. [PubMed:7884273 ]
- Loza-Tavera H: Monoterpenes in essential oils. Biosynthesis and properties. Adv Exp Med Biol. 1999;464:49-62. [PubMed:10335385 ]
- Barnard GF, Popjak G: Human liver prenyltransferase and its characterization. Biochim Biophys Acta. 1981 Sep 15;661(1):87-99. [PubMed:7295734 ]
- Pont F, Luciani B, Belmant C, Fournie JJ: Characterization of phosphoantigens by high-performance anion-exchange chromatography-electrospray ionization ion trap mass spectrometry and nanoelectrospray ionization ion trap mass spectrometry. Anal Chem. 2001 Aug 1;73(15):3562-9. [PubMed:11510819 ]
- Smit A, Mushegian A: Biosynthesis of isoprenoids via mevalonate in Archaea: the lost pathway. Genome Res. 2000 Oct;10(10):1468-84. [PubMed:11042147 ]
|
|---|