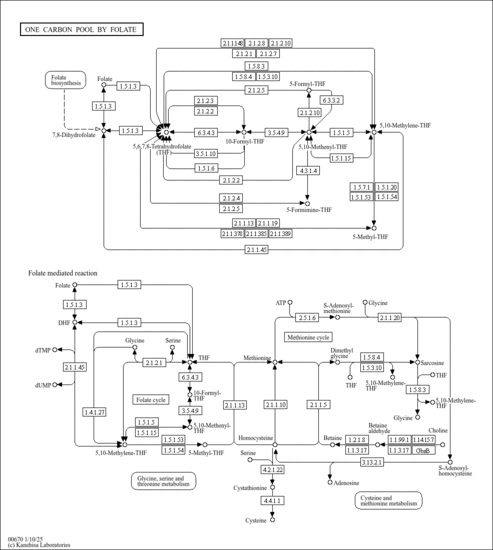
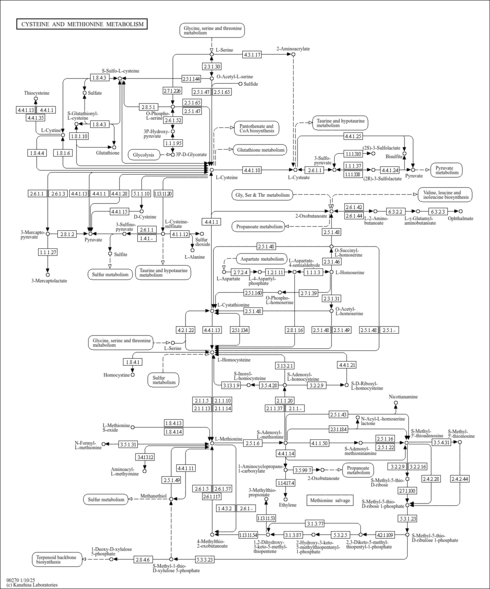
| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| 5-Methyltetrahydrofolic acid,1TMS,isomer #1 | CN1C2=C(N=C(N)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1 | 4750.7 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,1TMS,isomer #2 | CN1C2=C(N=C(N)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1 | 4745.4 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,1TMS,isomer #3 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1 | 4912.5 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,1TMS,isomer #4 | CN1C2=C(N=C(N)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1)[Si](C)(C)C | 4733.9 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,1TMS,isomer #5 | CN1C2=C(N=C(N)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1 | 4706.3 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,1TMS,isomer #6 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1 | 4628.1 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,1TMS,isomer #7 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1 | 4797.5 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,2TMS,isomer #1 | CN1C2=C(N=C(N)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1 | 4563.1 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,2TMS,isomer #10 | CN1C2=C(N=C(N)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 4515.6 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,2TMS,isomer #11 | CN1C2=C(N=C(N)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 4543.5 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,2TMS,isomer #12 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1 | 4756.8 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,2TMS,isomer #13 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1 | 4810.1 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,2TMS,isomer #14 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1 | 4647.9 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,2TMS,isomer #15 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1)[Si](C)(C)C | 4726.4 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,2TMS,isomer #16 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1 | 4711.1 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,2TMS,isomer #17 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1)[Si](C)(C)C | 4639.8 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,2TMS,isomer #18 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1)[Si](C)(C)C | 4426.1 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,2TMS,isomer #19 | CN1C2=C(N=C(N)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4478.2 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,2TMS,isomer #2 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1 | 4704.1 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,2TMS,isomer #20 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1 | 4605.2 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,2TMS,isomer #21 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1 | 4393.5 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,2TMS,isomer #22 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1 | 4586.7 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,2TMS,isomer #3 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1 | 4627.6 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,2TMS,isomer #4 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1 | 4427.1 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,2TMS,isomer #5 | CN1C2=C(N=C(N)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1)[Si](C)(C)C | 4518.5 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,2TMS,isomer #6 | CN1C2=C(N=C(N)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1 | 4517.2 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,2TMS,isomer #7 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1 | 4711.6 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,2TMS,isomer #8 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1 | 4636.4 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,2TMS,isomer #9 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1 | 4431.3 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #1 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1 | 4567.0 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #1 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1 | 4054.3 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #1 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1 | 6486.9 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #10 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1 | 4518.1 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #10 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1 | 4113.3 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #10 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1 | 6514.7 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #11 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1 | 4453.2 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #11 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1 | 4149.5 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #11 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1 | 6416.1 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #12 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1)[Si](C)(C)C | 4475.8 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #12 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1)[Si](C)(C)C | 4113.9 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #12 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1)[Si](C)(C)C | 6478.2 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #13 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1 | 4445.1 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #13 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1 | 4083.9 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #13 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1 | 6545.6 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #14 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1)[Si](C)(C)C | 4245.6 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #14 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1)[Si](C)(C)C | 4104.7 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #14 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1)[Si](C)(C)C | 6145.1 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #15 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1 | 4224.3 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #15 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1 | 4060.6 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #15 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1 | 6198.1 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #16 | CN1C2=C(N=C(N)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4318.3 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #16 | CN1C2=C(N=C(N)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4002.3 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #16 | CN1C2=C(N=C(N)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 6253.8 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #17 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1 | 4575.8 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #17 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1 | 4196.6 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #17 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1 | 6331.9 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #18 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1 | 4657.2 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #18 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1 | 4171.9 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #18 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1 | 6399.9 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #19 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1 | 4482.6 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #19 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1 | 4136.5 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #19 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1 | 6154.9 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #2 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1 | 4492.4 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #2 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1 | 4047.0 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #2 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1 | 6559.9 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #20 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 4542.4 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #20 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 4095.6 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #20 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 6366.0 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #21 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 4538.2 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #21 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 4084.7 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #21 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 6469.6 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #22 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1 | 4457.4 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #22 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1 | 4099.3 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #22 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1 | 6343.5 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #23 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 4484.0 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #23 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 4074.3 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #23 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 6400.0 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #24 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 4463.8 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #24 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 4049.3 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #24 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 6499.6 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #25 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 4244.7 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #25 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 4056.4 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #25 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 6072.5 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #26 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 4239.7 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #26 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 4021.4 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #26 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 6156.8 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #27 | CN1C2=C(N=C(N)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4334.4 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #27 | CN1C2=C(N=C(N)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 3977.9 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #27 | CN1C2=C(N=C(N)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 6208.3 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #28 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1 | 4754.5 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #28 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1 | 4366.1 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #28 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1 | 6395.0 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #29 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1 | 4530.1 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #29 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1 | 4304.1 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #29 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1 | 6087.7 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #3 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1 | 4263.4 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #3 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1 | 4027.8 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #3 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1 | 6222.3 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #30 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1)[Si](C)(C)C | 4612.1 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #30 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1)[Si](C)(C)C | 4295.9 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #30 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1)[Si](C)(C)C | 6286.8 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #31 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1 | 4535.6 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #31 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1 | 4237.4 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #31 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1 | 6364.7 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #32 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1 | 4638.6 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #32 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1 | 4284.9 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #32 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1 | 6151.9 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #33 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1)[Si](C)(C)C | 4677.7 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #33 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1)[Si](C)(C)C | 4254.9 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #33 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1)[Si](C)(C)C | 6340.7 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #34 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1 | 4625.7 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #34 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1 | 4219.5 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #34 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1 | 6424.1 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #35 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1)[Si](C)(C)C | 4480.9 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #35 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1)[Si](C)(C)C | 4236.7 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #35 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1)[Si](C)(C)C | 6106.7 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #36 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1 | 4451.3 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #36 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1 | 4184.2 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #36 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1 | 6176.5 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #37 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4509.2 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #37 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4137.0 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #37 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 6383.2 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #38 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1)[Si](C)(C)C | 4462.1 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #38 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1)[Si](C)(C)C | 4205.5 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #38 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1)[Si](C)(C)C | 6274.5 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #39 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4433.0 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #39 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4109.1 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #39 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 6393.4 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #4 | CN1C2=C(N=C(N)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 4383.0 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #4 | CN1C2=C(N=C(N)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 3961.6 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #4 | CN1C2=C(N=C(N)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 6277.8 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #40 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4207.1 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #40 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4093.9 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #40 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 6054.7 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #41 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1 | 4401.2 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #41 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1 | 4130.4 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #41 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1 | 6338.0 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #5 | CN1C2=C(N=C(N)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 4399.9 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #5 | CN1C2=C(N=C(N)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 3965.1 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #5 | CN1C2=C(N=C(N)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 6370.3 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #6 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1 | 4572.4 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #6 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1 | 4239.1 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #6 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1 | 6409.2 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #7 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1 | 4649.9 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #7 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1 | 4214.9 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #7 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1 | 6475.2 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #8 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1 | 4477.9 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #8 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1 | 4188.9 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #8 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1 | 6223.2 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #9 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1)[Si](C)(C)C | 4538.4 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #9 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1)[Si](C)(C)C | 4132.5 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TMS,isomer #9 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1)[Si](C)(C)C | 6442.2 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #1 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1 | 4466.3 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #1 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1 | 4101.8 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #1 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1 | 5949.7 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #10 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 4127.9 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #10 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 3979.1 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #10 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 5920.8 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #11 | CN1C2=C(N=C(N)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4249.2 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #11 | CN1C2=C(N=C(N)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 3898.8 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #11 | CN1C2=C(N=C(N)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 5933.4 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #12 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1 | 4621.5 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #12 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1 | 4276.2 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #12 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1 | 5997.1 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #13 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1 | 4395.1 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #13 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1 | 4231.4 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #13 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1 | 5744.4 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #14 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1)[Si](C)(C)C | 4447.1 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #14 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1)[Si](C)(C)C | 4172.4 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #14 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1)[Si](C)(C)C | 5910.3 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #15 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1 | 4384.8 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #15 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1 | 4165.8 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #15 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1 | 5981.3 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #16 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1 | 4516.6 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #16 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1 | 4189.9 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #16 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1 | 5814.5 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #17 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1)[Si](C)(C)C | 4535.6 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #17 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1)[Si](C)(C)C | 4126.4 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #17 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1)[Si](C)(C)C | 5978.5 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #18 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1 | 4479.0 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #18 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1 | 4130.9 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #18 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1 | 6052.1 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #19 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1)[Si](C)(C)C | 4340.7 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #19 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1)[Si](C)(C)C | 4117.7 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #19 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1)[Si](C)(C)C | 5771.6 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #2 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1 | 4540.3 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #2 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1 | 4072.1 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #2 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1 | 6022.4 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #20 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1 | 4317.5 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #20 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1 | 4109.1 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #20 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1 | 5838.5 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #21 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4369.3 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #21 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4027.9 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #21 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 6013.9 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #22 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1)[Si](C)(C)C | 4328.3 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #22 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1)[Si](C)(C)C | 4104.6 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #22 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1)[Si](C)(C)C | 6020.4 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #23 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1 | 4279.4 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #23 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1 | 4082.4 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #23 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1 | 6083.0 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #24 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4313.9 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #24 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4023.2 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #24 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 6104.9 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #25 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4109.3 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #25 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4013.7 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #25 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 5804.6 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #26 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1 | 4619.7 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #26 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1 | 4249.3 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #26 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1 | 5935.1 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #27 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1 | 4386.8 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #27 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1 | 4198.1 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #27 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1 | 5688.8 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #28 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 4446.5 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #28 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 4152.9 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #28 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 5849.6 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #29 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 4401.0 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #29 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 4148.0 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #29 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 5946.9 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #3 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1 | 4352.4 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #3 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1 | 4051.1 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #3 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1 | 5797.3 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #30 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1 | 4514.9 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #30 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1 | 4160.6 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #30 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1 | 5761.4 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #31 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 4539.7 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #31 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 4106.2 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #31 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 5920.9 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #32 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 4503.1 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #32 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 4114.6 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #32 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 6020.1 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #33 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 4350.6 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #33 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 4087.5 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #33 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 5717.1 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #34 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 4328.2 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #34 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 4086.0 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #34 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 5808.5 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #35 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4385.5 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #35 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4017.9 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #35 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 5981.0 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #36 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 4327.9 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #36 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 4077.2 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #36 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 5961.1 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #37 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 4296.8 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #37 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 4059.7 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #37 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 6050.4 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #38 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4329.4 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #38 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4012.5 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #38 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 6069.5 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #39 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4117.0 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #39 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 3996.8 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #39 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 5771.8 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #4 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 4417.4 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #4 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 3963.7 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #4 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 5991.8 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #40 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1 | 4600.5 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #40 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1 | 4353.5 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #40 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1 | 5736.4 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #41 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1)[Si](C)(C)C | 4634.8 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #41 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1)[Si](C)(C)C | 4324.2 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #41 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1)[Si](C)(C)C | 5881.1 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #42 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1 | 4541.9 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #42 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1 | 4293.7 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #42 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1 | 5962.3 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #43 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1)[Si](C)(C)C | 4407.2 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #43 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1)[Si](C)(C)C | 4288.0 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #43 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1)[Si](C)(C)C | 5640.7 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #44 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1 | 4340.1 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #44 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1 | 4246.6 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #44 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1 | 5711.9 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #45 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4401.4 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #45 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4208.5 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #45 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 5867.2 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #46 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1)[Si](C)(C)C | 4514.2 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #46 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1)[Si](C)(C)C | 4233.7 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #46 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1)[Si](C)(C)C | 5705.1 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #47 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1 | 4462.2 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #47 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1 | 4210.1 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #47 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1 | 5779.0 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #48 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4491.3 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #48 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4153.1 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #48 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 5932.9 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #49 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4317.7 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #49 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4138.1 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #49 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 5729.5 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #5 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 4417.2 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #5 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 4000.8 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #5 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 6075.3 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #50 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4277.8 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #50 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4120.1 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #50 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 5945.5 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #6 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1 | 4349.3 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #6 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1 | 4041.1 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #6 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1 | 6104.8 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #7 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 4374.4 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #7 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 3971.5 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #7 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 6130.2 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #8 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 4347.6 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #8 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 3993.3 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #8 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 6220.6 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #9 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 4129.1 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #9 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 3971.1 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,4TMS,isomer #9 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 5837.9 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #1 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1 | 4500.8 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #1 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1 | 4157.2 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #1 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1 | 5576.1 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #10 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4321.5 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #10 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 3924.1 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #10 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 5624.2 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #11 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 4252.4 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #11 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 3999.3 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #11 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 5758.2 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #12 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 4216.4 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #12 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 4017.6 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #12 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 5844.4 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #13 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4274.0 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #13 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 3935.3 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #13 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 5839.2 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #14 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4063.2 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #14 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 3929.0 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #14 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 5560.4 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #15 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1 | 4482.8 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #15 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1 | 4278.6 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #15 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1 | 5425.7 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #16 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1)[Si](C)(C)C | 4498.3 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #16 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1)[Si](C)(C)C | 4199.6 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #16 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1)[Si](C)(C)C | 5540.8 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #17 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1 | 4423.9 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #17 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1 | 4219.5 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #17 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1 | 5620.4 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #18 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1)[Si](C)(C)C | 4327.3 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #18 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1)[Si](C)(C)C | 4179.7 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #18 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1)[Si](C)(C)C | 5337.4 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #19 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1 | 4250.9 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #19 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1 | 4188.4 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #19 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1 | 5410.8 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #2 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1 | 4312.0 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #2 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1 | 4126.3 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #2 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1 | 5360.4 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #20 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4319.7 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #20 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4113.4 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #20 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 5536.0 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #21 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1)[Si](C)(C)C | 4406.3 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #21 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1)[Si](C)(C)C | 4116.3 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #21 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1)[Si](C)(C)C | 5407.6 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #22 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1 | 4357.6 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #22 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1 | 4139.7 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #22 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1 | 5484.6 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #23 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4383.5 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #23 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4052.2 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #23 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 5607.5 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #24 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4244.2 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #24 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4049.7 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #24 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 5429.5 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #25 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4218.0 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #25 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4053.1 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #25 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 5737.3 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #26 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1 | 4468.6 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #26 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1 | 4261.5 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #26 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1 | 5382.5 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #27 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 4494.1 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #27 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 4192.5 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #27 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 5493.2 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #28 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 4430.6 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #28 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 4213.0 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #28 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 5594.0 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #29 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 4322.8 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #29 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 4163.6 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #29 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 5294.6 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #3 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 4379.9 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #3 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 4035.0 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #3 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 5500.2 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #30 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 4257.0 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #30 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 4176.2 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #30 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 5385.6 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #31 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4326.5 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #31 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4110.3 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #31 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 5508.6 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #32 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 4415.4 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #32 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 4105.7 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #32 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 5363.9 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #33 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 4367.7 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #33 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 4128.2 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #33 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 5460.5 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #34 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4404.1 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #34 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4052.5 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #34 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 5581.1 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #35 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4248.6 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #35 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4044.2 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #35 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 5404.6 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #36 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4229.9 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #36 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4046.6 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #36 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 5708.5 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #37 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1)[Si](C)(C)C | 4492.9 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #37 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1)[Si](C)(C)C | 4320.3 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #37 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1)[Si](C)(C)C | 5339.3 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #38 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1 | 4414.8 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #38 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1 | 4315.8 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #38 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1 | 5417.5 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #39 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4460.8 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #39 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4255.7 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #39 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 5525.4 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #4 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 4340.4 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #4 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 4073.5 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #4 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 5591.2 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #40 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4281.6 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #40 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4226.7 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #40 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 5325.8 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #41 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4378.4 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #41 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4165.3 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #41 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 5390.3 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #5 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1 | 4406.7 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #5 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1 | 4082.3 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #5 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1 | 5435.0 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #6 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 4417.8 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #6 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 3981.3 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #6 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 5573.7 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #7 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 4396.3 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #7 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 4031.8 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #7 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 5667.2 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #8 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 4264.9 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #8 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 3982.9 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #8 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 5384.1 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #9 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 4234.1 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #9 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 4019.8 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,5TMS,isomer #9 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 5470.6 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #1 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1 | 4394.2 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #1 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1 | 4195.8 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #1 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1 | 5082.3 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #10 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4189.3 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #10 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 3971.0 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #10 | CN1C2=C(N=C(N[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 5101.2 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #11 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4184.0 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #11 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 3988.2 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #11 | CN1C2=C(N=C(N)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 5518.7 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #12 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1)[Si](C)(C)C | 4423.4 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #12 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1)[Si](C)(C)C | 4226.6 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #12 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)C=C1)[Si](C)(C)C | 5054.4 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #13 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1 | 4341.9 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #13 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1 | 4258.3 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #13 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1 | 5141.8 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #14 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4398.3 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #14 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4159.3 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #14 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 5218.2 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #15 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4222.3 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #15 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4152.0 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #15 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 5034.8 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #16 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4314.9 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #16 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4085.2 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #16 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 5119.4 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #17 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 4420.9 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #17 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 4220.6 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #17 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 5025.3 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #18 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 4341.0 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #18 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 4251.7 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #18 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 5122.9 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #19 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4405.1 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #19 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4162.1 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #19 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 5198.3 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #2 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 4446.5 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #2 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 4084.6 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #2 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 5173.3 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #20 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4228.6 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #20 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4149.5 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #20 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 5016.7 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #21 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4323.4 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #21 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4084.9 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #21 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 5101.3 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #22 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4384.7 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #22 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4280.7 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #22 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C)C=C1)[Si](C)(C)C | 5065.6 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #3 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 4379.0 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #3 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 4137.8 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #3 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 5272.2 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #4 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 4267.9 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #4 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 4079.0 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #4 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 4988.9 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #5 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 4198.0 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #5 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 4119.5 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #5 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 5080.8 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #6 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4307.1 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #6 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4021.4 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #6 | CN1C2=C(N=C(N([Si](C)(C)C)[Si](C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 5180.1 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #7 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 4342.0 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #7 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 4018.6 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #7 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)C=C1)[Si](C)(C)C | 5068.4 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #8 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 4290.0 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #8 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 4070.7 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #8 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)N([Si](C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1 | 5162.4 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #9 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 4348.5 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #9 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 3967.1 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,6TMS,isomer #9 | CN1C2=C(N=C(N[Si](C)(C)C)N([Si](C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C)C(=O)O[Si](C)(C)C)[Si](C)(C)C)C=C1)[Si](C)(C)C | 5262.9 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,1TBDMS,isomer #1 | CN1C2=C(N=C(N)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)C=C1 | 5004.6 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,1TBDMS,isomer #2 | CN1C2=C(N=C(N)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)C=C1 | 4980.1 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,1TBDMS,isomer #3 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1 | 5093.5 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,1TBDMS,isomer #4 | CN1C2=C(N=C(N)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1)[Si](C)(C)C(C)(C)C | 4958.3 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,1TBDMS,isomer #5 | CN1C2=C(N=C(N)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C)C=C1 | 4934.2 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,1TBDMS,isomer #6 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C(C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1 | 4886.0 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,1TBDMS,isomer #7 | CN1C2=C(N=C(N)N([Si](C)(C)C(C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1 | 5001.5 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,2TBDMS,isomer #1 | CN1C2=C(N=C(N)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C)C=C1 | 5035.6 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,2TBDMS,isomer #10 | CN1C2=C(N=C(N)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)C=C1)[Si](C)(C)C(C)(C)C | 4994.7 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,2TBDMS,isomer #11 | CN1C2=C(N=C(N)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)C=C1 | 5001.6 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,2TBDMS,isomer #12 | CN1C2=C(N=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1 | 5162.8 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,2TBDMS,isomer #13 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)N([Si](C)(C)C(C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1 | 5216.3 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,2TBDMS,isomer #14 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)[NH]C2=O)N([Si](C)(C)C(C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1 | 5041.7 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,2TBDMS,isomer #15 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1)[Si](C)(C)C(C)(C)C | 5131.4 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,2TBDMS,isomer #16 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C)C=C1 | 5103.1 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,2TBDMS,isomer #17 | CN1C2=C(N=C(N)N([Si](C)(C)C(C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1)[Si](C)(C)C(C)(C)C | 5087.8 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,2TBDMS,isomer #18 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C(C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1)[Si](C)(C)C(C)(C)C | 4892.3 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,2TBDMS,isomer #19 | CN1C2=C(N=C(N)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C)C=C1)[Si](C)(C)C(C)(C)C | 4935.7 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,2TBDMS,isomer #2 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)C=C1 | 5147.4 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,2TBDMS,isomer #20 | CN1C2=C(N=C(N)N([Si](C)(C)C(C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C)C=C1 | 5043.1 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,2TBDMS,isomer #21 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C(C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C)C=C1 | 4858.3 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,2TBDMS,isomer #22 | CN1C2=C(N=C(N)N([Si](C)(C)C(C)(C)C)C2=O)N([Si](C)(C)C(C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1 | 5061.1 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,2TBDMS,isomer #3 | CN1C2=C(N=C(N)N([Si](C)(C)C(C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)C=C1 | 5116.4 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,2TBDMS,isomer #4 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C(C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)C=C1 | 4912.7 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,2TBDMS,isomer #5 | CN1C2=C(N=C(N)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)C=C1)[Si](C)(C)C(C)(C)C | 5008.4 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,2TBDMS,isomer #6 | CN1C2=C(N=C(N)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)[Si](C)(C)C(C)(C)C)C=C1 | 4999.2 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,2TBDMS,isomer #7 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)C=C1 | 5135.5 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,2TBDMS,isomer #8 | CN1C2=C(N=C(N)N([Si](C)(C)C(C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)C=C1 | 5101.6 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,2TBDMS,isomer #9 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C(C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)C=C1 | 4913.5 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #1 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C)C=C1 | 5123.9 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #1 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C)C=C1 | 4643.1 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #1 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C)C=C1 | 6263.7 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #10 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)[Si](C)(C)C(C)(C)C)C=C1 | 5090.4 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #10 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)[Si](C)(C)C(C)(C)C)C=C1 | 4655.7 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #10 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)[Si](C)(C)C(C)(C)C)C=C1 | 6274.8 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #11 | CN1C2=C(N=C(N)N([Si](C)(C)C(C)(C)C)C2=O)N([Si](C)(C)C(C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)C=C1 | 5107.9 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #11 | CN1C2=C(N=C(N)N([Si](C)(C)C(C)(C)C)C2=O)N([Si](C)(C)C(C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)C=C1 | 4720.8 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #11 | CN1C2=C(N=C(N)N([Si](C)(C)C(C)(C)C)C2=O)N([Si](C)(C)C(C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)C=C1 | 6225.8 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #12 | CN1C2=C(N=C(N)N([Si](C)(C)C(C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)C=C1)[Si](C)(C)C(C)(C)C | 5134.2 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #12 | CN1C2=C(N=C(N)N([Si](C)(C)C(C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)C=C1)[Si](C)(C)C(C)(C)C | 4665.3 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #12 | CN1C2=C(N=C(N)N([Si](C)(C)C(C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)C=C1)[Si](C)(C)C(C)(C)C | 6237.8 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #13 | CN1C2=C(N=C(N)N([Si](C)(C)C(C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)[Si](C)(C)C(C)(C)C)C=C1 | 5068.8 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #13 | CN1C2=C(N=C(N)N([Si](C)(C)C(C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)[Si](C)(C)C(C)(C)C)C=C1 | 4629.3 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #13 | CN1C2=C(N=C(N)N([Si](C)(C)C(C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)[Si](C)(C)C(C)(C)C)C=C1 | 6285.9 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #14 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C(C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)C=C1)[Si](C)(C)C(C)(C)C | 4896.2 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #14 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C(C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)C=C1)[Si](C)(C)C(C)(C)C | 4609.6 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #14 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C(C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)C=C1)[Si](C)(C)C(C)(C)C | 6039.8 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #15 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C(C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)[Si](C)(C)C(C)(C)C)C=C1 | 4857.0 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #15 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C(C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)[Si](C)(C)C(C)(C)C)C=C1 | 4567.8 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #15 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C(C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)[Si](C)(C)C(C)(C)C)C=C1 | 6083.7 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #16 | CN1C2=C(N=C(N)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)[Si](C)(C)C(C)(C)C)C=C1)[Si](C)(C)C(C)(C)C | 4954.2 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #16 | CN1C2=C(N=C(N)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)[Si](C)(C)C(C)(C)C)C=C1)[Si](C)(C)C(C)(C)C | 4518.0 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #16 | CN1C2=C(N=C(N)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)[Si](C)(C)C(C)(C)C)C=C1)[Si](C)(C)C(C)(C)C | 6095.1 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #17 | CN1C2=C(N=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)C=C1 | 5174.3 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #17 | CN1C2=C(N=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)C=C1 | 4767.6 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #17 | CN1C2=C(N=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)C=C1 | 6125.5 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #18 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)N([Si](C)(C)C(C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)C=C1 | 5224.2 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #18 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)N([Si](C)(C)C(C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)C=C1 | 4767.9 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #18 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)N([Si](C)(C)C(C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)C=C1 | 6166.3 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #19 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)[NH]C2=O)N([Si](C)(C)C(C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)C=C1 | 5024.5 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #19 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)[NH]C2=O)N([Si](C)(C)C(C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)C=C1 | 4717.3 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #19 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)[NH]C2=O)N([Si](C)(C)C(C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)C=C1 | 6045.4 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #2 | CN1C2=C(N=C(N)N([Si](C)(C)C(C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C)C=C1 | 5105.7 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #2 | CN1C2=C(N=C(N)N([Si](C)(C)C(C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C)C=C1 | 4637.8 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #2 | CN1C2=C(N=C(N)N([Si](C)(C)C(C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C)C=C1 | 6301.8 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #20 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)C=C1)[Si](C)(C)C(C)(C)C | 5127.4 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #20 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)C=C1)[Si](C)(C)C(C)(C)C | 4657.4 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #20 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)C=C1)[Si](C)(C)C(C)(C)C | 6197.2 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #21 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)C=C1 | 5091.9 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #21 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)C=C1 | 4652.1 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #21 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)C=C1 | 6259.9 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #22 | CN1C2=C(N=C(N)N([Si](C)(C)C(C)(C)C)C2=O)N([Si](C)(C)C(C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)C=C1 | 5094.7 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #22 | CN1C2=C(N=C(N)N([Si](C)(C)C(C)(C)C)C2=O)N([Si](C)(C)C(C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)C=C1 | 4689.0 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #22 | CN1C2=C(N=C(N)N([Si](C)(C)C(C)(C)C)C2=O)N([Si](C)(C)C(C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)C=C1 | 6181.2 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #23 | CN1C2=C(N=C(N)N([Si](C)(C)C(C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)C=C1)[Si](C)(C)C(C)(C)C | 5118.2 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #23 | CN1C2=C(N=C(N)N([Si](C)(C)C(C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)C=C1)[Si](C)(C)C(C)(C)C | 4637.6 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #23 | CN1C2=C(N=C(N)N([Si](C)(C)C(C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)C=C1)[Si](C)(C)C(C)(C)C | 6191.8 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #24 | CN1C2=C(N=C(N)N([Si](C)(C)C(C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)C=C1 | 5071.5 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #24 | CN1C2=C(N=C(N)N([Si](C)(C)C(C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)C=C1 | 4619.5 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #24 | CN1C2=C(N=C(N)N([Si](C)(C)C(C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)C=C1 | 6265.4 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #25 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C(C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)C=C1)[Si](C)(C)C(C)(C)C | 4881.1 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #25 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C(C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)C=C1)[Si](C)(C)C(C)(C)C | 4571.4 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #25 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C(C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)C=C1)[Si](C)(C)C(C)(C)C | 5995.3 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #26 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C(C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)C=C1 | 4861.5 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #26 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C(C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)C=C1 | 4549.2 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #26 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C(C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)C=C1 | 6063.4 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #27 | CN1C2=C(N=C(N)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)C=C1)[Si](C)(C)C(C)(C)C | 4953.2 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #27 | CN1C2=C(N=C(N)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)C=C1)[Si](C)(C)C(C)(C)C | 4514.5 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #27 | CN1C2=C(N=C(N)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)C=C1)[Si](C)(C)C(C)(C)C | 6073.6 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #28 | CN1C2=C(N=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N([Si](C)(C)C(C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1 | 5305.5 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #28 | CN1C2=C(N=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N([Si](C)(C)C(C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1 | 4905.1 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #28 | CN1C2=C(N=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)N([Si](C)(C)C(C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1 | 6128.5 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #29 | CN1C2=C(N=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[NH]C2=O)N([Si](C)(C)C(C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1 | 5088.9 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #29 | CN1C2=C(N=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[NH]C2=O)N([Si](C)(C)C(C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1 | 4833.0 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #29 | CN1C2=C(N=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[NH]C2=O)N([Si](C)(C)C(C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1 | 5959.0 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #3 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C(C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C)C=C1 | 4895.0 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #3 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C(C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C)C=C1 | 4581.1 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #3 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C(C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C)C=C1 | 6098.4 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #30 | CN1C2=C(N=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1)[Si](C)(C)C(C)(C)C | 5178.5 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #30 | CN1C2=C(N=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1)[Si](C)(C)C(C)(C)C | 4795.0 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #30 | CN1C2=C(N=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1)[Si](C)(C)C(C)(C)C | 6084.4 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #31 | CN1C2=C(N=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C)C=C1 | 5122.4 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #31 | CN1C2=C(N=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C)C=C1 | 4770.1 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #31 | CN1C2=C(N=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C)C=C1 | 6132.6 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #32 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)N([Si](C)(C)C(C)(C)C)C2=O)N([Si](C)(C)C(C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1 | 5223.5 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #32 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)N([Si](C)(C)C(C)(C)C)C2=O)N([Si](C)(C)C(C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1 | 4829.2 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #32 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)N([Si](C)(C)C(C)(C)C)C2=O)N([Si](C)(C)C(C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1 | 6002.7 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #33 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)N([Si](C)(C)C(C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1)[Si](C)(C)C(C)(C)C | 5267.4 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #33 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)N([Si](C)(C)C(C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1)[Si](C)(C)C(C)(C)C | 4799.9 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #33 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)N([Si](C)(C)C(C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1)[Si](C)(C)C(C)(C)C | 6126.5 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #34 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)N([Si](C)(C)C(C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C)C=C1 | 5189.3 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #34 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)N([Si](C)(C)C(C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C)C=C1 | 4769.4 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #34 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)N([Si](C)(C)C(C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C)C=C1 | 6176.0 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #35 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)[NH]C2=O)N([Si](C)(C)C(C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1)[Si](C)(C)C(C)(C)C | 5062.9 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #35 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)[NH]C2=O)N([Si](C)(C)C(C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1)[Si](C)(C)C(C)(C)C | 4745.6 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #35 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)[NH]C2=O)N([Si](C)(C)C(C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1)[Si](C)(C)C(C)(C)C | 6016.8 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #36 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)[NH]C2=O)N([Si](C)(C)C(C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C)C=C1 | 5005.9 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #36 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)[NH]C2=O)N([Si](C)(C)C(C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C)C=C1 | 4721.8 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #36 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)[NH]C2=O)N([Si](C)(C)C(C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C)C=C1 | 6060.5 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #37 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C)C=C1)[Si](C)(C)C(C)(C)C | 5098.4 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #37 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C)C=C1)[Si](C)(C)C(C)(C)C | 4676.4 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #37 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C)C=C1)[Si](C)(C)C(C)(C)C | 6200.8 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #38 | CN1C2=C(N=C(N)N([Si](C)(C)C(C)(C)C)C2=O)N([Si](C)(C)C(C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1)[Si](C)(C)C(C)(C)C | 5118.6 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #38 | CN1C2=C(N=C(N)N([Si](C)(C)C(C)(C)C)C2=O)N([Si](C)(C)C(C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1)[Si](C)(C)C(C)(C)C | 4720.7 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #38 | CN1C2=C(N=C(N)N([Si](C)(C)C(C)(C)C)C2=O)N([Si](C)(C)C(C)(C)C)CC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O)C(=O)O)C=C1)[Si](C)(C)C(C)(C)C | 6129.5 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #39 | CN1C2=C(N=C(N)N([Si](C)(C)C(C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C)C=C1)[Si](C)(C)C(C)(C)C | 5079.2 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #39 | CN1C2=C(N=C(N)N([Si](C)(C)C(C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C)C=C1)[Si](C)(C)C(C)(C)C | 4646.2 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #39 | CN1C2=C(N=C(N)N([Si](C)(C)C(C)(C)C)C2=O)NCC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C)C=C1)[Si](C)(C)C(C)(C)C | 6184.1 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #4 | CN1C2=C(N=C(N)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C)C=C1)[Si](C)(C)C(C)(C)C | 4991.0 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #4 | CN1C2=C(N=C(N)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C)C=C1)[Si](C)(C)C(C)(C)C | 4516.4 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #4 | CN1C2=C(N=C(N)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C)C=C1)[Si](C)(C)C(C)(C)C | 6113.6 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #40 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C(C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C)C=C1)[Si](C)(C)C(C)(C)C | 4859.8 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #40 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C(C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C)C=C1)[Si](C)(C)C(C)(C)C | 4578.0 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #40 | CN1C2=C(N=C(N)[NH]C2=O)N([Si](C)(C)C(C)(C)C)CC1CN(C1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C)C=C1)[Si](C)(C)C(C)(C)C | 5987.6 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #41 | CN1C2=C(N=C(N)N([Si](C)(C)C(C)(C)C)C2=O)N([Si](C)(C)C(C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C)C=C1 | 5059.1 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #41 | CN1C2=C(N=C(N)N([Si](C)(C)C(C)(C)C)C2=O)N([Si](C)(C)C(C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C)C=C1 | 4689.1 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #41 | CN1C2=C(N=C(N)N([Si](C)(C)C(C)(C)C)C2=O)N([Si](C)(C)C(C)(C)C)CC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O)C(=O)O)[Si](C)(C)C(C)(C)C)C=C1 | 6177.3 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #5 | CN1C2=C(N=C(N)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)C=C1 | 5005.8 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #5 | CN1C2=C(N=C(N)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)C=C1 | 4507.3 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #5 | CN1C2=C(N=C(N)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N([C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)C=C1 | 6184.3 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #6 | CN1C2=C(N=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)C=C1 | 5197.1 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #6 | CN1C2=C(N=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)C=C1 | 4793.5 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #6 | CN1C2=C(N=C(N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[NH]C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)C=C1 | 6165.7 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #7 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)N([Si](C)(C)C(C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)C=C1 | 5236.4 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #7 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)N([Si](C)(C)C(C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)C=C1 | 4790.0 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #7 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)N([Si](C)(C)C(C)(C)C)C2=O)NCC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)C=C1 | 6203.8 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #8 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)[NH]C2=O)N([Si](C)(C)C(C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)C=C1 | 5040.4 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #8 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)[NH]C2=O)N([Si](C)(C)C(C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)C=C1 | 4747.2 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #8 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)[NH]C2=O)N([Si](C)(C)C(C)(C)C)CC1CNC1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)C=C1 | 6082.4 | Standard polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #9 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)C=C1)[Si](C)(C)C(C)(C)C | 5137.0 | Semi standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #9 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)C=C1)[Si](C)(C)C(C)(C)C | 4678.1 | Standard non polar | 33892256 |
| 5-Methyltetrahydrofolic acid,3TBDMS,isomer #9 | CN1C2=C(N=C(N[Si](C)(C)C(C)(C)C)[NH]C2=O)NCC1CN(C1=CC=C(C(=O)N[C@H](CCC(=O)O[Si](C)(C)C(C)(C)C)C(=O)O)C=C1)[Si](C)(C)C(C)(C)C | 6236.2 | Standard polar | 33892256 |