| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Expected but not Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2022-03-07 02:49:08 UTC |
|---|
| HMDB ID | HMDB0001206 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Acetyl-CoA |
|---|
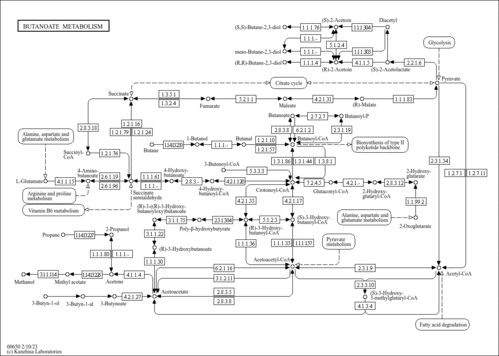
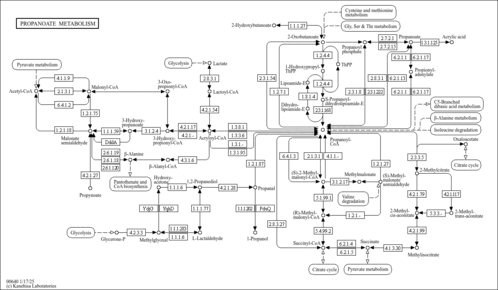
| Description | The main function of coenzyme A is to carry acyl groups (such as the acetyl group) or thioesters. Acetyl-CoA is an important molecule itself. It is the precursor to HMG CoA, which is a vital component in cholesterol and ketone synthesis. (wikipedia). acetyl CoA participates in the biosynthesis of fatty acids and sterols, in the oxidation of fatty acids and in the metabolism of many amino acids. It also acts as a biological acetylating agent. |
|---|
| Structure | CC(=O)SCCNC(=O)CCNC(=O)[C@H](O)C(C)(C)COP(O)(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP(O)(O)=O)N1C=NC2=C1N=CN=C2N InChI=1S/C23H38N7O17P3S/c1-12(31)51-7-6-25-14(32)4-5-26-21(35)18(34)23(2,3)9-44-50(41,42)47-49(39,40)43-8-13-17(46-48(36,37)38)16(33)22(45-13)30-11-29-15-19(24)27-10-28-20(15)30/h10-11,13,16-18,22,33-34H,4-9H2,1-3H3,(H,25,32)(H,26,35)(H,39,40)(H,41,42)(H2,24,27,28)(H2,36,37,38)/t13-,16-,17-,18+,22-/m1/s1 |
|---|
| Synonyms | | Value | Source |
|---|
| AcCoA | ChEBI | | Acetyl coenzyme A | ChEBI | | S-Acetyl-CoA | ChEBI | | S-Acetyl-coenzyme A | ChEBI | | Acetyl CoA | MeSH | | CoA, Acetyl | MeSH | | coenzyme A, Acetyl | MeSH | | Ac-CoA | HMDB | | Ac-coenzyme A | HMDB | | Ac-S-CoA | HMDB | | Ac-S-coenzyme A | HMDB | | Acetyl-coenzyme A | HMDB | | Acetyl-S-CoA | HMDB | | Acetyl-S-coenzyme A | HMDB | | Acetylcoenzyme A | HMDB | | S-Acetate CoA | HMDB | | S-Acetate coenzyme A | HMDB | | S-Acetyl coenzyme A | HMDB |
|
|---|
| Chemical Formula | C23H38N7O17P3S |
|---|
| Average Molecular Weight | 809.571 |
|---|
| Monoisotopic Molecular Weight | 809.125773051 |
|---|
| IUPAC Name | {[(2R,3S,4R,5R)-2-({[({[(3R)-3-[(2-{[2-(acetylsulfanyl)ethyl]carbamoyl}ethyl)carbamoyl]-3-hydroxy-2,2-dimethylpropoxy](hydroxy)phosphoryl}oxy)(hydroxy)phosphoryl]oxy}methyl)-5-(6-amino-9H-purin-9-yl)-4-hydroxyoxolan-3-yl]oxy}phosphonic acid |
|---|
| Traditional Name | acetyl-CoA |
|---|
| CAS Registry Number | 72-89-9 |
|---|
| SMILES | CC(=O)SCCNC(=O)CCNC(=O)[C@H](O)C(C)(C)COP(O)(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP(O)(O)=O)N1C=NC2=C1N=CN=C2N |
|---|
| InChI Identifier | InChI=1S/C23H38N7O17P3S/c1-12(31)51-7-6-25-14(32)4-5-26-21(35)18(34)23(2,3)9-44-50(41,42)47-49(39,40)43-8-13-17(46-48(36,37)38)16(33)22(45-13)30-11-29-15-19(24)27-10-28-20(15)30/h10-11,13,16-18,22,33-34H,4-9H2,1-3H3,(H,25,32)(H,26,35)(H,39,40)(H,41,42)(H2,24,27,28)(H2,36,37,38)/t13-,16-,17-,18+,22-/m1/s1 |
|---|
| InChI Key | ZSLZBFCDCINBPY-ZSJPKINUSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as acyl coas. These are organic compounds containing a coenzyme A substructure linked to an acyl chain. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acyl thioesters |
|---|
| Direct Parent | Acyl CoAs |
|---|
| Alternative Parents | |
|---|
| Substituents | - Coenzyme a or derivatives
- Purine ribonucleoside 3',5'-bisphosphate
- Purine ribonucleoside bisphosphate
- Purine ribonucleoside diphosphate
- Ribonucleoside 3'-phosphate
- Pentose phosphate
- Pentose-5-phosphate
- Beta amino acid or derivatives
- Glycosyl compound
- N-glycosyl compound
- 6-aminopurine
- Monosaccharide phosphate
- Organic pyrophosphate
- Pentose monosaccharide
- Imidazopyrimidine
- Purine
- Monoalkyl phosphate
- Aminopyrimidine
- Imidolactam
- N-acyl-amine
- N-substituted imidazole
- Organic phosphoric acid derivative
- Monosaccharide
- Pyrimidine
- Alkyl phosphate
- Fatty amide
- Phosphoric acid ester
- Tetrahydrofuran
- Imidazole
- Azole
- Heteroaromatic compound
- Carbothioic s-ester
- Secondary alcohol
- Thiocarboxylic acid ester
- Carboxamide group
- Secondary carboxylic acid amide
- Amino acid or derivatives
- Sulfenyl compound
- Thiocarboxylic acid or derivatives
- Organoheterocyclic compound
- Azacycle
- Oxacycle
- Carboxylic acid derivative
- Organosulfur compound
- Organic oxygen compound
- Hydrocarbon derivative
- Carbonyl group
- Organic nitrogen compound
- Primary amine
- Organopnictogen compound
- Organic oxide
- Organooxygen compound
- Organonitrogen compound
- Alcohol
- Amine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Experimental Collision Cross Sections |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Retention Times Underivatized| Chromatographic Method | Retention Time | Reference |
|---|
| Measured using a Waters Acquity ultraperformance liquid chromatography (UPLC) ethylene-bridged hybrid (BEH) C18 column (100 mm × 2.1 mm; 1.7 μmparticle diameter). Predicted by Afia on May 17, 2022. Predicted by Afia on May 17, 2022. | 3.31 minutes | 32390414 | | Predicted by Siyang on May 30, 2022 | 10.0771 minutes | 33406817 | | Predicted by Siyang using ReTip algorithm on June 8, 2022 | 9.39 minutes | 32390414 | | AjsUoB = Accucore 150 Amide HILIC with 10mM Ammonium Formate, 0.1% Formic Acid | 520.4 seconds | 40023050 | | Fem_Long = Waters ACQUITY UPLC HSS T3 C18 with Water:MeOH and 0.1% Formic Acid | 1056.4 seconds | 40023050 | | Fem_Lipids = Ascentis Express C18 with (60:40 water:ACN):(90:10 IPA:ACN) and 10mM NH4COOH + 0.1% Formic Acid | 171.9 seconds | 40023050 | | Life_Old = Waters ACQUITY UPLC BEH C18 with Water:(20:80 acetone:ACN) and 0.1% Formic Acid | 87.4 seconds | 40023050 | | Life_New = RP Waters ACQUITY UPLC HSS T3 C18 with Water:(30:70 MeOH:ACN) and 0.1% Formic Acid | 162.5 seconds | 40023050 | | RIKEN = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 74.1 seconds | 40023050 | | Eawag_XBridgeC18 = XBridge C18 3.5u 2.1x50 mm with Water:MeOH and 0.1% Formic Acid | 350.2 seconds | 40023050 | | BfG_NTS_RP1 =Agilent Zorbax Eclipse Plus C18 (2.1 mm x 150 mm, 3.5 um) with Water:ACN and 0.1% Formic Acid | 412.9 seconds | 40023050 | | HILIC_BDD_2 = Merck SeQuant ZIC-HILIC with ACN(0.1% formic acid):water(16 mM ammonium formate) | 851.7 seconds | 40023050 | | UniToyama_Atlantis = RP Waters Atlantis T3 (2.1 x 150 mm, 5 um) with ACN:Water and 0.1% Formic Acid | 628.4 seconds | 40023050 | | BDD_C18 = Hypersil Gold 1.9µm C18 with Water:ACN and 0.1% Formic Acid | 270.5 seconds | 40023050 | | UFZ_Phenomenex = Kinetex Core-Shell C18 2.6 um, 3.0 x 100 mm, Phenomenex with Water:MeOH and 0.1% Formic Acid | 659.3 seconds | 40023050 | | SNU_RIKEN_POS = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 212.6 seconds | 40023050 | | RPMMFDA = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 233.4 seconds | 40023050 | | MTBLS87 = Merck SeQuant ZIC-pHILIC column with ACN:Water and :ammonium carbonate | 504.3 seconds | 40023050 | | KI_GIAR_zic_HILIC_pH2_7 = Merck SeQuant ZIC-HILIC with ACN:Water and 0.1% FA | 370.5 seconds | 40023050 | | Meister zic-pHILIC pH9.3 = Merck SeQuant ZIC-pHILIC column with ACN:Water 5mM NH4Ac pH9.3 and 5mM ammonium acetate in water | 477.6 seconds | 40023050 |
Predicted Kovats Retention IndicesUnderivatized |
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - Acetyl-CoA GC-MS (TMS_1_1) - 70eV, Positive | Not Available | 2021-10-19 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Acetyl-CoA GC-MS (TMS_1_2) - 70eV, Positive | Not Available | 2021-10-19 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Acetyl-CoA GC-MS (TMS_1_3) - 70eV, Positive | Not Available | 2021-10-19 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Acetyl-CoA GC-MS (TMS_1_4) - 70eV, Positive | Not Available | 2021-10-19 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Acetyl-CoA GC-MS (TMS_1_5) - 70eV, Positive | Not Available | 2021-10-19 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Acetyl-CoA GC-MS (TMS_1_6) - 70eV, Positive | Not Available | 2021-10-19 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Acetyl-CoA GC-MS (TMS_1_7) - 70eV, Positive | Not Available | 2021-10-19 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Acetyl-CoA GC-MS (TMS_1_8) - 70eV, Positive | Not Available | 2021-10-19 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetyl-CoA LC-ESI-ITFT 35V, positive-QTOF | splash10-0fb9-0035910000-cecfaf54528fc3ef9f00 | 2020-07-21 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetyl-CoA LC-ESI-ITFT 35V, positive-QTOF | splash10-0ufr-0005900000-491971eb7a5c2b327554 | 2020-07-21 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetyl-CoA LC-ESI-ITFT 35V, negative-QTOF | splash10-0a4i-0900000000-1b259612c3897ed851f0 | 2020-07-21 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetyl-CoA LC-ESI-ITFT 35V, negative-QTOF | splash10-08i0-0001901200-8d4f5232c27f2875cf8b | 2020-07-21 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetyl-CoA LC-ESI-ITFT 35V, negative-QTOF | splash10-08i0-0000901200-33f8d3ced8c83336df0c | 2020-07-21 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetyl-CoA 33V, Negative-QTOF | splash10-0a4i-1101800090-5031dc04edf8ba0f3afb | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetyl-CoA 33V, Negative-QTOF | splash10-0a4i-1101900080-6691464da5a030f7d086 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetyl-CoA 33V, Negative-QTOF | splash10-0a4i-1101900080-8a1491c56fc1331c5df1 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Acetyl-CoA 33V, Negative-QTOF | splash10-0a4i-1101900080-9ec3eecc20f19a822002 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Acetyl-CoA 10V, Positive-QTOF | splash10-000i-1901000300-57c996f08055dba75dd7 | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Acetyl-CoA 20V, Positive-QTOF | splash10-000i-0902000000-dffb00601bfc54014ae4 | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Acetyl-CoA 40V, Positive-QTOF | splash10-000i-2901000000-155f0890adf4c76dca85 | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Acetyl-CoA 10V, Negative-QTOF | splash10-0arr-6820231930-984ae0f98e0d17e4a7fc | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Acetyl-CoA 20V, Negative-QTOF | splash10-003r-3910100000-87da6b6d742efbc6e74a | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Acetyl-CoA 40V, Negative-QTOF | splash10-057i-5900000000-8701decc3b2311880b97 | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Acetyl-CoA 10V, Negative-QTOF | splash10-0a4i-0000000090-fd17a06b039262f96463 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Acetyl-CoA 20V, Negative-QTOF | splash10-05vx-9100203430-9e39d5da3afdd3a15f91 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Acetyl-CoA 40V, Negative-QTOF | splash10-00b9-9101401200-719cb55952a06d96d3c8 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Acetyl-CoA 10V, Positive-QTOF | splash10-03di-0000000090-02de4c2ee732e6d50b2d | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Acetyl-CoA 20V, Positive-QTOF | splash10-01p9-1901002440-79ee9e61778bd08a750f | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Acetyl-CoA 40V, Positive-QTOF | splash10-0udi-0209000000-84f7f68fc2e83a8ae770 | 2021-09-23 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-25 | Wishart Lab | View Spectrum |
|
|---|
| General References | - Blank ML, Smith ZL, Fitzgerald V, Snyder F: The CoA-independent transacylase in PAF biosynthesis: tissue distribution and molecular species selectivity. Biochim Biophys Acta. 1995 Feb 9;1254(3):295-301. [PubMed:7857969 ]
- Wysocki SJ, Wilkinson SP, Hahnel R, Wong CY, Panegyres PK: 3-Hydroxy-3-methylglutaric aciduria, combined with 3-methylglutaconic aciduria. Clin Chim Acta. 1976 Aug 2;70(3):399-406. [PubMed:947633 ]
- Al-Buheissi SZ, Patel HR, Meinl W, Hewer A, Bryan RL, Glatt H, Miller RA, Phillips DH: N-Acetyltransferase and sulfotransferase activity in human prostate: potential for carcinogen activation. Pharmacogenet Genomics. 2006 Jun;16(6):391-9. [PubMed:16708048 ]
- Michno A, Skibowska A, Raszeja-Specht A, Cwikowska J, Szutowicz A: The role of adenosine triphosphate citrate lyase in the metabolism of acetyl coenzyme a and function of blood platelets in diabetes mellitus. Metabolism. 2004 Jan;53(1):66-72. [PubMed:14681844 ]
- Griffin MJ, Sul HS: Insulin regulation of fatty acid synthase gene transcription: roles of USF and SREBP-1c. IUBMB Life. 2004 Oct;56(10):595-600. [PubMed:15814457 ]
- Putman CT, Spriet LL, Hultman E, Dyck DJ, Heigenhauser GJ: Skeletal muscle pyruvate dehydrogenase activity during acetate infusion in humans. Am J Physiol. 1995 May;268(5 Pt 1):E1007-17. [PubMed:7762627 ]
- Szutowicz A, Tomaszewicz M, Jankowska A, Madziar B, Bielarczyk H: [Mechanisms of selective vulnerability of cholinergic neurons to neurotoxic stimuli]. Postepy Hig Med Dosw. 1999;53(2):263-75. [PubMed:10355292 ]
- Ingebretsen OC, Bakken AM, Farstad M: The content of coenzyme A, acetyl-CoA and long-chain acyl-CoA in human blood platelets. Clin Chim Acta. 1982 Dec 23;126(3):307-13. [PubMed:7151284 ]
- Michno A, Raszeja-Specht A, Jankowska-Kulawy A, Pawelczyk T, Szutowicz A: Effect of L-carnitine on acetyl-CoA content and activity of blood platelets in healthy and diabetic persons. Clin Chem. 2005 Sep;51(9):1673-82. Epub 2005 Jul 14. [PubMed:16020499 ]
- Constantin-Teodosiu D, Peirce NS, Fox J, Greenhaff PL: Muscle pyruvate availability can limit the flux, but not activation, of the pyruvate dehydrogenase complex during submaximal exercise in humans. J Physiol. 2004 Dec 1;561(Pt 2):647-55. Epub 2004 Oct 7. [PubMed:15579544 ]
- Crystal HA, Davies P: Cortical substance P-like immunoreactivity in cases of Alzheimer's disease and senile dementia of the Alzheimer type. J Neurochem. 1982 Jun;38(6):1781-4. [PubMed:6176686 ]
- Evans MK, Savasi I, Heigenhauser GJ, Spriet LL: Effects of acetate infusion and hyperoxia on muscle substrate phosphorylation after onset of moderate exercise. Am J Physiol Endocrinol Metab. 2001 Dec;281(6):E1144-50. [PubMed:11701427 ]
- Peters SJ: Regulation of PDH activity and isoform expression: diet and exercise. Biochem Soc Trans. 2003 Dec;31(Pt 6):1274-80. [PubMed:14641042 ]
- Roe CR, Sweetman L, Roe DS, David F, Brunengraber H: Treatment of cardiomyopathy and rhabdomyolysis in long-chain fat oxidation disorders using an anaplerotic odd-chain triglyceride. J Clin Invest. 2002 Jul;110(2):259-69. [PubMed:12122118 ]
- Skibowska A, Raszeja-Specht A, Szutowicz A: Platelet function and acetyl-coenzyme A metabolism in type 1 diabetes mellitus. Clin Chem Lab Med. 2003 Sep;41(9):1136-43. [PubMed:14598862 ]
- Girard J: [Contribution of free fatty acids to impairment of insulin secretion and action. mechanism of beta-cell lipotoxicity]. Med Sci (Paris). 2005 Dec;21 Spec No:19-25. [PubMed:16598900 ]
- Szutowicz A, Jankowska A, Tomaszewicz M: [Disturbances of glucose metabolism in epilepsy and other neurodegenerative diseases]. Neurol Neurochir Pol. 2000;34 Suppl 8:59-66. [PubMed:11780590 ]
- Spriet LL, MacLean DA, Dyck DJ, Hultman E, Cederblad G, Graham TE: Caffeine ingestion and muscle metabolism during prolonged exercise in humans. Am J Physiol. 1992 Jun;262(6 Pt 1):E891-8. [PubMed:1616022 ]
- Constantin-Teodosiu D, Carlin JI, Cederblad G, Harris RC, Hultman E: Acetyl group accumulation and pyruvate dehydrogenase activity in human muscle during incremental exercise. Acta Physiol Scand. 1991 Dec;143(4):367-72. [PubMed:1815472 ]
- Boden G, Jadali F, White J, Liang Y, Mozzoli M, Chen X, Coleman E, Smith C: Effects of fat on insulin-stimulated carbohydrate metabolism in normal men. J Clin Invest. 1991 Sep;88(3):960-6. [PubMed:1885781 ]
|
|---|