| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected and Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2022-03-07 02:48:59 UTC |
|---|
| HMDB ID | HMDB0000032 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 7-Dehydrocholesterol |
|---|
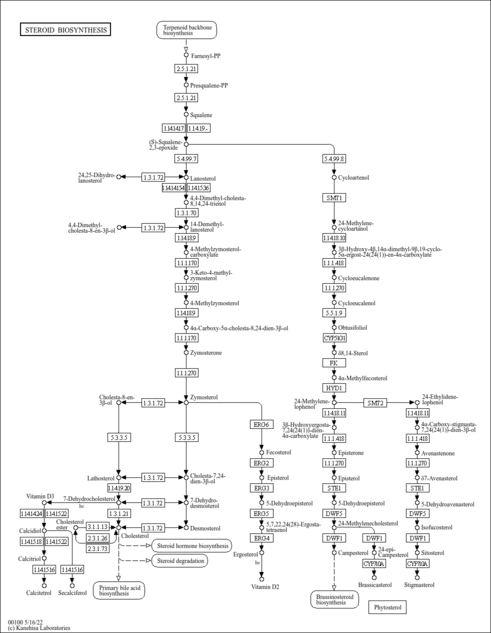
| Description | 7-Dehydrocholesterol (7-DHC), also known as provitamin D3 or 5,7-cholestadien-3-b-ol, belongs to the class of organic compounds known as cholesterols and derivatives. Cholesterols and derivatives are compounds containing a 3-hydroxylated cholestane core. Thus, 7-dehydrocholesterol is also classified as a sterol. 7-Dehydrocholesterol is known as a zoosterol, meaning that it is a sterol isolated from animals (to distinguish those sterols isolated from plants which are called phytosterols). 7-DHC functions in the serum as a cholesterol precursor and is photochemically converted to vitamin D3 in the skin. Therefore 7-DHC functions as provitamin-D3. The presence of 7-DHC in human skin enables humans and other mammals to manufacture vitamin D3 (cholecalciferol) from ultraviolet rays in the sun light, via an intermediate isomer pre-vitamin D3. 7-DHC absorbs UV light most effectively at wavelengths between 290 and 320 nm and, thus, the production of vitamin D3 will occur primarily at those wavelengths (PMID: 9625080 ). The two most important factors that govern the generation of pre-vitamin D3 are the quantity (intensity) and quality (appropriate wavelength) of the UVB irradiation reaching the 7-dehydrocholesterol deep in the stratum basale and stratum spinosum (PMID: 9625080 ). 7-DHC is also found in the milk of several mammalian species, including cows (PMID: 10999630 ; PMID: 225459 ). It was discovered by Nobel-laureate organic chemist Adolf Windaus. 7-DHC can be produced by animals and plants via different pathways (PMID: 23717318 ). It is not produced by fungi in significant amounts. 7-DHC is made by some algae and can also be produced by some bacteria. |
|---|
| Structure | [H][C@@]1(CC[C@@]2([H])C3=CC=C4C[C@@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCCC(C)C InChI=1S/C27H44O/c1-18(2)7-6-8-19(3)23-11-12-24-22-10-9-20-17-21(28)13-15-26(20,4)25(22)14-16-27(23,24)5/h9-10,18-19,21,23-25,28H,6-8,11-17H2,1-5H3/t19-,21+,23-,24+,25+,26+,27-/m1/s1 |
|---|
| Synonyms | | Value | Source |
|---|
| (3beta)-Cholesta-5,7-dien-3-ol | ChEBI | | 5,7-Cholestadien-3-beta-ol | ChEBI | | 5,7-Cholestadien-3beta-ol | ChEBI | | Provitamin D3 | ChEBI | | Cholesta-5,7-dien-3beta-ol | Kegg | | (3b)-Cholesta-5,7-dien-3-ol | Generator | | (3Β)-cholesta-5,7-dien-3-ol | Generator | | 5,7-Cholestadien-3-b-ol | Generator | | 5,7-Cholestadien-3-β-ol | Generator | | 5,7-Cholestadien-3b-ol | Generator | | 5,7-Cholestadien-3β-ol | Generator | | Cholesta-5,7-dien-3b-ol | Generator | | Cholesta-5,7-dien-3β-ol | Generator | | (-)-7-Dehydrocholesterol | HMDB | | 10,13-Dimethyl-17-(6-methylheptan-2-yl)-2,3,4,9,11,12,14,15,16,17-decahydro-1H-cyclopenta[a]phenanthren-3-ol | HMDB | | 17-(1,5-Dimethylhexyl)-10,13-dimethyl-2,3,4,9,10,11,12,13,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol | HMDB | | 5,7-Cholestandien-3-ol | HMDB | | 5,7-Cholestandien-3beta-ol | HMDB | | 7,8-Dehydro-cholesterol | HMDB | | 7,8-Didehydrocholesterol | HMDB | | 7-Dehydro-cholesterol | HMDB | | 7-Dehydrocholesterin | HMDB | | 7DHC | HMDB | | Cholesta-5,7-dien-3 beta -ol | HMDB | | Cholesta-5,7-dien-3-beta-ol | HMDB | | Cholesta-5,7-dien-3-ol | HMDB | | Dehydrocholesterin | HMDB | | Dehydrocholesterol | HMDB | | Delta5,7-Cholestadien-3beta-ol | HMDB | | Delta5,7-Cholesterol | HMDB | | Delta7-Cholesterol | HMDB | | Provitamin-D3 | HMDB | | Provitamin D(3) | HMDB | | Cholesta-5,7-dien-3 beta-ol | HMDB | | 7-Dehydrocholesterol, (3beta,10alpha)-isomer | HMDB | | 7-Dehydrocholesterol, (3beta,9beta,10alpha)-isomer | HMDB | | 7-DHC | HMDB | | 7-Dehydrocholesterol, (3alpha)-isomer | HMDB | | 7-Dehydrocholesterol, (3beta,9beta)-isomer | HMDB |
|
|---|
| Chemical Formula | C27H44O |
|---|
| Average Molecular Weight | 384.6377 |
|---|
| Monoisotopic Molecular Weight | 384.33921603 |
|---|
| IUPAC Name | (1S,2R,5S,11R,14R,15R)-2,15-dimethyl-14-[(2R)-6-methylheptan-2-yl]tetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-7,9-dien-5-ol |
|---|
| Traditional Name | (1S,2R,5S,11R,14R,15R)-2,15-dimethyl-14-[(2R)-6-methylheptan-2-yl]tetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-7,9-dien-5-ol |
|---|
| CAS Registry Number | 434-16-2 |
|---|
| SMILES | [H][C@@]1(CC[C@@]2([H])C3=CC=C4C[C@@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCCC(C)C |
|---|
| InChI Identifier | InChI=1S/C27H44O/c1-18(2)7-6-8-19(3)23-11-12-24-22-10-9-20-17-21(28)13-15-26(20,4)25(22)14-16-27(23,24)5/h9-10,18-19,21,23-25,28H,6-8,11-17H2,1-5H3/t19-,21+,23-,24+,25+,26+,27-/m1/s1 |
|---|
| InChI Key | UCTLRSWJYQTBFZ-DDPQNLDTSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as cholesterols and derivatives. Cholesterols and derivatives are compounds containing a 3-hydroxylated cholestane core. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Cholestane steroids |
|---|
| Direct Parent | Cholesterols and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cholesterol
- Cholesterol-skeleton
- 3-hydroxy-delta-5-steroid
- 3-hydroxy-delta-7-steroid
- 3-beta-hydroxysteroid
- 3-beta-hydroxy-delta-5-steroid
- 3-hydroxysteroid
- Hydroxysteroid
- Delta-7-steroid
- Delta-5-steroid
- Cyclic alcohol
- Secondary alcohol
- Alcohol
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | 150.5 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 1 mg/mL | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Not Available |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Retention Times Underivatized| Chromatographic Method | Retention Time | Reference |
|---|
| Measured using a Waters Acquity ultraperformance liquid chromatography (UPLC) ethylene-bridged hybrid (BEH) C18 column (100 mm × 2.1 mm; 1.7 μmparticle diameter). Predicted by Afia on May 17, 2022. Predicted by Afia on May 17, 2022. | 9.33 minutes | 32390414 | | Predicted by Siyang on May 30, 2022 | 27.7839 minutes | 33406817 | | Predicted by Siyang using ReTip algorithm on June 8, 2022 | 1.81 minutes | 32390414 | | Fem_Long = Waters ACQUITY UPLC HSS T3 C18 with Water:MeOH and 0.1% Formic Acid | 3662.2 seconds | 40023050 | | Fem_Lipids = Ascentis Express C18 with (60:40 water:ACN):(90:10 IPA:ACN) and 10mM NH4COOH + 0.1% Formic Acid | 873.2 seconds | 40023050 | | Life_Old = Waters ACQUITY UPLC BEH C18 with Water:(20:80 acetone:ACN) and 0.1% Formic Acid | 329.0 seconds | 40023050 | | Life_New = RP Waters ACQUITY UPLC HSS T3 C18 with Water:(30:70 MeOH:ACN) and 0.1% Formic Acid | 389.3 seconds | 40023050 | | RIKEN = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 683.5 seconds | 40023050 | | Eawag_XBridgeC18 = XBridge C18 3.5u 2.1x50 mm with Water:MeOH and 0.1% Formic Acid | 1271.0 seconds | 40023050 | | BfG_NTS_RP1 =Agilent Zorbax Eclipse Plus C18 (2.1 mm x 150 mm, 3.5 um) with Water:ACN and 0.1% Formic Acid | 1151.4 seconds | 40023050 | | HILIC_BDD_2 = Merck SeQuant ZIC-HILIC with ACN(0.1% formic acid):water(16 mM ammonium formate) | 103.0 seconds | 40023050 | | UniToyama_Atlantis = RP Waters Atlantis T3 (2.1 x 150 mm, 5 um) with ACN:Water and 0.1% Formic Acid | 2302.4 seconds | 40023050 | | BDD_C18 = Hypersil Gold 1.9µm C18 with Water:ACN and 0.1% Formic Acid | 724.8 seconds | 40023050 | | UFZ_Phenomenex = Kinetex Core-Shell C18 2.6 um, 3.0 x 100 mm, Phenomenex with Water:MeOH and 0.1% Formic Acid | 2302.2 seconds | 40023050 | | SNU_RIKEN_POS = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 840.4 seconds | 40023050 | | RPMMFDA = Waters ACQUITY UPLC BEH C18 with Water:ACN and 0.1% Formic Acid | 609.7 seconds | 40023050 | | MTBLS87 = Merck SeQuant ZIC-pHILIC column with ACN:Water and :ammonium carbonate | 392.8 seconds | 40023050 | | KI_GIAR_zic_HILIC_pH2_7 = Merck SeQuant ZIC-HILIC with ACN:Water and 0.1% FA | 796.0 seconds | 40023050 | | Meister zic-pHILIC pH9.3 = Merck SeQuant ZIC-pHILIC column with ACN:Water 5mM NH4Ac pH9.3 and 5mM ammonium acetate in water | 8.8 seconds | 40023050 |
Predicted Kovats Retention IndicesUnderivatizedDerivatized |
|---|
| General References | - Lehmann B, Genehr T, Knuschke P, Pietzsch J, Meurer M: UVB-induced conversion of 7-dehydrocholesterol to 1alpha,25-dihydroxyvitamin D3 in an in vitro human skin equivalent model. J Invest Dermatol. 2001 Nov;117(5):1179-85. [PubMed:11710930 ]
- Muzzin KB, Harper LF: Smith-Lemli-Opitz syndrome: a review, case report and dental implications. Spec Care Dentist. 2003;23(1):22-7. [PubMed:12887150 ]
- Paterson CR, Moody JP, Pennington CR: Skin content of 7-dehydrocholesterol in patients with malabsorption. Nutrition. 1997 Sep;13(9):771-3. [PubMed:9290088 ]
- Jira PE, Wevers RA, de Jong J, Rubio-Gozalbo E, Janssen-Zijlstra FS, van Heyst AF, Sengers RC, Smeitink JA: Simvastatin. A new therapeutic approach for Smith-Lemli-Opitz syndrome. J Lipid Res. 2000 Aug;41(8):1339-46. [PubMed:10946022 ]
- Johnson DW, ten Brink HJ, Jakobs C: A rapid screening procedure for cholesterol and dehydrocholesterol by electrospray ionization tandem mass spectrometry. J Lipid Res. 2001 Oct;42(10):1699-705. [PubMed:11590227 ]
- van Rooij A, Nijenhuis AA, Wijburg FA, Schutgens RB: Highly increased CSF concentrations of cholesterol precursors in Smith-Lemli-Opitz syndrome. J Inherit Metab Dis. 1997 Aug;20(4):578-80. [PubMed:9266395 ]
- Andersson HC, Frentz J, Martinez JE, Tuck-Muller CM, Bellizaire J: Adrenal insufficiency in Smith-Lemli-Opitz syndrome. Am J Med Genet. 1999 Feb 19;82(5):382-4. [PubMed:10069708 ]
- GarciaFuentes E, icioso Recio MV, del Castillo Acedo Del Olmo E, atas Jurado MM, Arana Aguera M, Lopez Lopez J: [Biochemical diagnosis of Smith-Lemli-Opitz syndrome in a patient with congenital adrenal hyperplasia]. An Esp Pediatr. 2000 Nov;53(5):482-7. [PubMed:11141372 ]
- Jezela-Stanek A, Malunowicz EM, Ciara E, Popowska E, Goryluk-Kozakiewicz B, Spodar K, Czerwiecka M, Jezuita J, Nowaczyk MJ, Krajewska-Walasek M: Maternal urinary steroid profiles in prenatal diagnosis of Smith-Lemli-Opitz syndrome: first patient series comparing biochemical and molecular studies. Clin Genet. 2006 Jan;69(1):77-85. [PubMed:16451140 ]
- Moody JP, Humphries CA, Allan SM, Paterson CR: Determination of 7-dehydrocholesterol in human skin by high-performance liquid chromatography. J Chromatogr. 1990 Aug 24;530(1):19-27. [PubMed:2277111 ]
- Simons K, Toomre D: Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000 Oct;1(1):31-9. [PubMed:11413487 ]
- Watson AD: Thematic review series: systems biology approaches to metabolic and cardiovascular disorders. Lipidomics: a global approach to lipid analysis in biological systems. J Lipid Res. 2006 Oct;47(10):2101-11. Epub 2006 Aug 10. [PubMed:16902246 ]
- Sethi JK, Vidal-Puig AJ: Thematic review series: adipocyte biology. Adipose tissue function and plasticity orchestrate nutritional adaptation. J Lipid Res. 2007 Jun;48(6):1253-62. Epub 2007 Mar 20. [PubMed:17374880 ]
- Lingwood D, Simons K: Lipid rafts as a membrane-organizing principle. Science. 2010 Jan 1;327(5961):46-50. doi: 10.1126/science.1174621. [PubMed:20044567 ]
- Dietschy JM, Turley SD: Thematic review series: brain Lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J Lipid Res. 2004 Aug;45(8):1375-97. [PubMed:15254070 ]
- O'Byrne SM, Blaner WS: Retinol and retinyl esters: biochemistry and physiology. J Lipid Res. 2013 Jul;54(7):1731-43. doi: 10.1194/jlr.R037648. Epub 2013 Apr 26. [PubMed:23625372 ]
- Norman AW: Sunlight, season, skin pigmentation, vitamin D, and 25-hydroxyvitamin D: integral components of the vitamin D endocrine system. Am J Clin Nutr. 1998 Jun;67(6):1108-10. doi: 10.1093/ajcn/67.6.1108. [PubMed:9625080 ]
- Gomis DB, Fernandez MP, Gutierrez Alvarez MD: Simultaneous determination of fat-soluble vitamins and provitamins in milk by microcolumn liquid chromatography. J Chromatogr A. 2000 Sep 1;891(1):109-14. doi: 10.1016/s0021-9673(00)00623-3. [PubMed:10999630 ]
- Adachi A, Kobayashi T: Identification of vitamin D3 and 7-dehydrocholesterol in cow's milk by gas chromatography-mass spectrometry and their quantitation by high-performance liquid chromatography. J Nutr Sci Vitaminol (Tokyo). 1979;25(2):67-78. doi: 10.3177/jnsv.25.67. [PubMed:225459 ]
- Japelt RB, Jakobsen J: Vitamin D in plants: a review of occurrence, analysis, and biosynthesis. Front Plant Sci. 2013 May 13;4:136. doi: 10.3389/fpls.2013.00136. eCollection 2013. [PubMed:23717318 ]
- Gunstone, Frank D., John L. Harwood, and Albert J. Dijkstra (2007). The lipid handbook with CD-ROM. CRC Press.
- Linda T. Welson (2006). Triglycerides and Cholesterol Research. Nova Science Publishers Inc..
|
|---|