| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected and Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2023-02-21 17:14:39 UTC |
|---|
| HMDB ID | HMDB0000247 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Sorbitol |
|---|
| Description | Sorbitol is a polyhydric alcohol with about half the sweetness of sucrose. Sorbitol occurs naturally and is also produced synthetically from glucose. It was formerly used as a diuretic and may still be used as a laxative and in irrigating solutions for some surgical procedures. It is also used in many manufacturing processes, as a pharmaceutical aid, and in several research applications. Ascorbic acid fermentation; in solution form for moisture-conditioning of cosmetic creams and lotions, toothpaste, tobacco, gelatin; bodying agent for paper, textiles, and liquid pharmaceuticals; softener for candy; sugar crystallization inhibitor; surfactants; urethane resins and rigid foams; plasticizer, stabilizer for vinyl resins; food additive (sweetener, humectant, emulsifier, thickener, anticaking agent); dietary supplement. (Hawley's Condensed Chemical Dictionary) Biological Source: Occurs widely in plants ranging from algae to the higher orders. Fruits of the plant family Rosaceae, which include apples, pears, cherries, apricots, contain appreciable amounts. Rich sources are the fruits of the Sorbus and Crataegus species Use/Importance: Used for manufacturing of sorbose, propylene glycol, ascorbic acid, resins, plasticizers and as antifreeze mixtures with glycerol or glycol. Tablet diluent, sweetening agent and humectant, other food uses. Sorbitol is used in photometric determination of Ru(VI) and Ru(VIII); in acid-base titration of borate (Dictionary of Organic Compounds). |
|---|
| Structure | OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO InChI=1S/C6H14O6/c7-1-3(9)5(11)6(12)4(10)2-8/h3-12H,1-2H2/t3-,4+,5-,6-/m1/s1 |
|---|
| Synonyms | | Value | Source |
|---|
| (-)-Sorbitol | ChEBI | | (2R,3R,4R,5S)-Hexane-1,2,3,4,5,6-hexol | ChEBI | | D-(-)-Sorbitol | ChEBI | | D-Sorbit | ChEBI | | D-SORBITOL | ChEBI | | e 420 | ChEBI | | e-420 | ChEBI | | e420 | ChEBI | | g-Ol | ChEBI | | GLC-Ol | ChEBI | | L-Gulitol | ChEBI | | D-Glucitol | Kegg | | Sorbitol 3% in plastic container | Kegg | | D-Sorbol | HMDB | | Diakarmon | HMDB | | Esasorb | HMDB | | Foodol D 70 | HMDB | | Glucarine | HMDB | | Glucitol | HMDB | | Karion | HMDB | | Karion instant | HMDB | | Kyowa powder 50m | HMDB | | Multitol | HMDB | | Neosorb | HMDB | | Neosorb 20/60dC | HMDB | | Neosorb 70/02 | HMDB | | Neosorb 70/70 | HMDB | | Neosorb p 20/60 | HMDB | | Neosorb p 60 | HMDB | | Neosorb p 60W | HMDB | | Nivitin | HMDB | | Resulax | HMDB | | Sionit | HMDB | | Sionit K | HMDB | | Sionite | HMDB | | Sionon | HMDB | | Siosan | HMDB | | Sorbex m | HMDB | | Sorbex R | HMDB | | Sorbex RP | HMDB | | Sorbex S | HMDB | | Sorbex X | HMDB | | Sorbilande | HMDB | | Sorbilax | HMDB | | Sorbit | HMDB | | Sorbit D 70 | HMDB | | Sorbit D-powder | HMDB | | Sorbit DP | HMDB | | Sorbit DP 50 | HMDB | | Sorbit kyowa powder 50m | HMDB | | Sorbit L 70 | HMDB | | Sorbit S | HMDB | | Sorbit T 70 | HMDB | | Sorbit W 70 | HMDB | | Sorbit W-powder | HMDB | | Sorbit W-powder 50 | HMDB | | Sorbit WP | HMDB | | Sorbite | HMDB | | Sorbitol F | HMDB | | Sorbitol FK | HMDB | | Sorbitol FP | HMDB | | Sorbitol S | HMDB | | Sorbitol syrup C | HMDB | | Sorbitur | HMDB | | Sorbo | HMDB | | Sorbogem 712 | HMDB | | Sorbol | HMDB | | Sorbostyl | HMDB | | Medefield brand OF sorbitol | HMDB | | Sorbitol pfizer brand | HMDB | | Yal | HMDB | | Klysma sorbit | HMDB | | Medevac | HMDB | | Trommsdorff brand OF sorbitol | HMDB | | Baxter brand OF sorbitol | HMDB | | Pfizer brand OF sorbitol | HMDB |
|
|---|
| Chemical Formula | C6H14O6 |
|---|
| Average Molecular Weight | 182.1718 |
|---|
| Monoisotopic Molecular Weight | 182.07903818 |
|---|
| IUPAC Name | (2R,3R,4R,5S)-hexane-1,2,3,4,5,6-hexol |
|---|
| Traditional Name | D-sorbitol |
|---|
| CAS Registry Number | 50-70-4 |
|---|
| SMILES | OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO |
|---|
| InChI Identifier | InChI=1S/C6H14O6/c7-1-3(9)5(11)6(12)4(10)2-8/h3-12H,1-2H2/t3-,4+,5-,6-/m1/s1 |
|---|
| InChI Key | FBPFZTCFMRRESA-JGWLITMVSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as sugar alcohols. These are hydrogenated forms of carbohydrate in which the carbonyl group (aldehyde or ketone, reducing sugar) has been reduced to a primary or secondary hydroxyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Sugar alcohols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Sugar alcohol
- Monosaccharide
- Secondary alcohol
- Polyol
- Hydrocarbon derivative
- Primary alcohol
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Not Available | Not Available |
|---|
| Physical Properties |
|---|
| State | Liquid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | 11 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 2750 mg/mL | Not Available | | LogP | -2.20 | SANGSTER (1994) |
|
|---|
| Experimental Chromatographic Properties | Experimental Collision Cross Sections |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| Sorbitol,1TMS,isomer #1 | C[Si](C)(C)OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO | 1776.6 | Semi standard non polar | 33892256 | | Sorbitol,1TMS,isomer #2 | C[Si](C)(C)O[C@@H](CO)[C@@H](O)[C@H](O)[C@H](O)CO | 1732.3 | Semi standard non polar | 33892256 | | Sorbitol,1TMS,isomer #3 | C[Si](C)(C)O[C@H]([C@@H](O)CO)[C@H](O)[C@H](O)CO | 1715.4 | Semi standard non polar | 33892256 | | Sorbitol,1TMS,isomer #4 | C[Si](C)(C)O[C@H]([C@H](O)CO)[C@H](O)[C@@H](O)CO | 1715.4 | Semi standard non polar | 33892256 | | Sorbitol,1TMS,isomer #5 | C[Si](C)(C)O[C@H](CO)[C@@H](O)[C@H](O)[C@@H](O)CO | 1732.3 | Semi standard non polar | 33892256 | | Sorbitol,1TMS,isomer #6 | C[Si](C)(C)OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)CO | 1776.6 | Semi standard non polar | 33892256 | | Sorbitol,2TMS,isomer #1 | C[Si](C)(C)OC[C@H](O[Si](C)(C)C)[C@@H](O)[C@H](O)[C@H](O)CO | 1810.5 | Semi standard non polar | 33892256 | | Sorbitol,2TMS,isomer #10 | C[Si](C)(C)O[C@H]([C@@H](O)CO)[C@H](O[Si](C)(C)C)[C@H](O)CO | 1751.7 | Semi standard non polar | 33892256 | | Sorbitol,2TMS,isomer #11 | C[Si](C)(C)O[C@H](CO)[C@@H](O)[C@H](O[Si](C)(C)C)[C@@H](O)CO | 1778.2 | Semi standard non polar | 33892256 | | Sorbitol,2TMS,isomer #12 | C[Si](C)(C)OC[C@@H](O)[C@@H](O)[C@H](O[Si](C)(C)C)[C@@H](O)CO | 1794.2 | Semi standard non polar | 33892256 | | Sorbitol,2TMS,isomer #13 | C[Si](C)(C)O[C@H]([C@@H](CO)O[Si](C)(C)C)[C@H](O)[C@@H](O)CO | 1776.7 | Semi standard non polar | 33892256 | | Sorbitol,2TMS,isomer #14 | C[Si](C)(C)OC[C@@H](O)[C@@H](O[Si](C)(C)C)[C@H](O)[C@@H](O)CO | 1791.6 | Semi standard non polar | 33892256 | | Sorbitol,2TMS,isomer #15 | C[Si](C)(C)OC[C@@H](O[Si](C)(C)C)[C@@H](O)[C@H](O)[C@@H](O)CO | 1810.5 | Semi standard non polar | 33892256 | | Sorbitol,2TMS,isomer #2 | C[Si](C)(C)OC[C@H](O)[C@@H](O[Si](C)(C)C)[C@H](O)[C@H](O)CO | 1791.6 | Semi standard non polar | 33892256 | | Sorbitol,2TMS,isomer #3 | C[Si](C)(C)OC[C@H](O)[C@@H](O)[C@H](O[Si](C)(C)C)[C@H](O)CO | 1794.2 | Semi standard non polar | 33892256 | | Sorbitol,2TMS,isomer #4 | C[Si](C)(C)OC[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O[Si](C)(C)C | 1824.6 | Semi standard non polar | 33892256 | | Sorbitol,2TMS,isomer #5 | C[Si](C)(C)OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO[Si](C)(C)C | 1833.2 | Semi standard non polar | 33892256 | | Sorbitol,2TMS,isomer #6 | C[Si](C)(C)O[C@@H](CO)[C@@H](O[Si](C)(C)C)[C@H](O)[C@H](O)CO | 1776.7 | Semi standard non polar | 33892256 | | Sorbitol,2TMS,isomer #7 | C[Si](C)(C)O[C@@H](CO)[C@@H](O)[C@H](O[Si](C)(C)C)[C@H](O)CO | 1778.2 | Semi standard non polar | 33892256 | | Sorbitol,2TMS,isomer #8 | C[Si](C)(C)O[C@@H](CO)[C@@H](O)[C@H](O)[C@@H](CO)O[Si](C)(C)C | 1801.6 | Semi standard non polar | 33892256 | | Sorbitol,2TMS,isomer #9 | C[Si](C)(C)OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](CO)O[Si](C)(C)C | 1824.6 | Semi standard non polar | 33892256 | | Sorbitol,3TMS,isomer #1 | C[Si](C)(C)OC[C@H](O[Si](C)(C)C)[C@@H](O[Si](C)(C)C)[C@H](O)[C@H](O)CO | 1833.7 | Semi standard non polar | 33892256 | | Sorbitol,3TMS,isomer #10 | C[Si](C)(C)OC[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO[Si](C)(C)C)O[Si](C)(C)C | 1875.8 | Semi standard non polar | 33892256 | | Sorbitol,3TMS,isomer #11 | C[Si](C)(C)O[C@@H](CO)[C@@H](O[Si](C)(C)C)[C@H](O[Si](C)(C)C)[C@H](O)CO | 1833.0 | Semi standard non polar | 33892256 | | Sorbitol,3TMS,isomer #12 | C[Si](C)(C)O[C@@H](CO)[C@@H](O[Si](C)(C)C)[C@H](O)[C@@H](CO)O[Si](C)(C)C | 1834.9 | Semi standard non polar | 33892256 | | Sorbitol,3TMS,isomer #13 | C[Si](C)(C)OC[C@@H](O)[C@@H](O)[C@H](O[Si](C)(C)C)[C@H](CO)O[Si](C)(C)C | 1855.8 | Semi standard non polar | 33892256 | | Sorbitol,3TMS,isomer #14 | C[Si](C)(C)O[C@@H](CO)[C@@H](O)[C@H](O[Si](C)(C)C)[C@@H](CO)O[Si](C)(C)C | 1834.9 | Semi standard non polar | 33892256 | | Sorbitol,3TMS,isomer #15 | C[Si](C)(C)OC[C@@H](O)[C@@H](O[Si](C)(C)C)[C@H](O)[C@H](CO)O[Si](C)(C)C | 1844.6 | Semi standard non polar | 33892256 | | Sorbitol,3TMS,isomer #16 | C[Si](C)(C)OC[C@@H](O[Si](C)(C)C)[C@@H](O)[C@H](O)[C@H](CO)O[Si](C)(C)C | 1863.9 | Semi standard non polar | 33892256 | | Sorbitol,3TMS,isomer #17 | C[Si](C)(C)O[C@H](CO)[C@@H](O[Si](C)(C)C)[C@H](O[Si](C)(C)C)[C@@H](O)CO | 1833.0 | Semi standard non polar | 33892256 | | Sorbitol,3TMS,isomer #18 | C[Si](C)(C)OC[C@@H](O)[C@@H](O[Si](C)(C)C)[C@H](O[Si](C)(C)C)[C@@H](O)CO | 1836.3 | Semi standard non polar | 33892256 | | Sorbitol,3TMS,isomer #19 | C[Si](C)(C)OC[C@@H](O[Si](C)(C)C)[C@@H](O)[C@H](O[Si](C)(C)C)[C@@H](O)CO | 1852.4 | Semi standard non polar | 33892256 | | Sorbitol,3TMS,isomer #2 | C[Si](C)(C)OC[C@H](O[Si](C)(C)C)[C@@H](O)[C@H](O[Si](C)(C)C)[C@H](O)CO | 1852.4 | Semi standard non polar | 33892256 | | Sorbitol,3TMS,isomer #20 | C[Si](C)(C)OC[C@@H](O[Si](C)(C)C)[C@@H](O[Si](C)(C)C)[C@H](O)[C@@H](O)CO | 1833.7 | Semi standard non polar | 33892256 | | Sorbitol,3TMS,isomer #3 | C[Si](C)(C)OC[C@H](O[Si](C)(C)C)[C@@H](O)[C@H](O)[C@@H](CO)O[Si](C)(C)C | 1863.9 | Semi standard non polar | 33892256 | | Sorbitol,3TMS,isomer #4 | C[Si](C)(C)OC[C@H](O[Si](C)(C)C)[C@@H](O)[C@H](O)[C@H](O)CO[Si](C)(C)C | 1875.8 | Semi standard non polar | 33892256 | | Sorbitol,3TMS,isomer #5 | C[Si](C)(C)OC[C@H](O)[C@@H](O[Si](C)(C)C)[C@H](O[Si](C)(C)C)[C@H](O)CO | 1836.3 | Semi standard non polar | 33892256 | | Sorbitol,3TMS,isomer #6 | C[Si](C)(C)OC[C@H](O)[C@@H](O[Si](C)(C)C)[C@H](O)[C@@H](CO)O[Si](C)(C)C | 1844.6 | Semi standard non polar | 33892256 | | Sorbitol,3TMS,isomer #7 | C[Si](C)(C)OC[C@H](O)[C@@H](O[Si](C)(C)C)[C@H](O)[C@H](O)CO[Si](C)(C)C | 1859.1 | Semi standard non polar | 33892256 | | Sorbitol,3TMS,isomer #8 | C[Si](C)(C)OC[C@H](O)[C@@H](O)[C@H](O[Si](C)(C)C)[C@@H](CO)O[Si](C)(C)C | 1855.8 | Semi standard non polar | 33892256 | | Sorbitol,3TMS,isomer #9 | C[Si](C)(C)OC[C@H](O)[C@@H](O)[C@H](O[Si](C)(C)C)[C@H](O)CO[Si](C)(C)C | 1859.1 | Semi standard non polar | 33892256 | | Sorbitol,4TMS,isomer #1 | C[Si](C)(C)OC[C@H](O[Si](C)(C)C)[C@@H](O[Si](C)(C)C)[C@H](O[Si](C)(C)C)[C@H](O)CO | 1880.0 | Semi standard non polar | 33892256 | | Sorbitol,4TMS,isomer #10 | C[Si](C)(C)OC[C@H](O)[C@@H](O)[C@H](O[Si](C)(C)C)[C@@H](CO[Si](C)(C)C)O[Si](C)(C)C | 1894.2 | Semi standard non polar | 33892256 | | Sorbitol,4TMS,isomer #11 | C[Si](C)(C)O[C@@H](CO)[C@@H](O[Si](C)(C)C)[C@H](O[Si](C)(C)C)[C@@H](CO)O[Si](C)(C)C | 1852.9 | Semi standard non polar | 33892256 | | Sorbitol,4TMS,isomer #12 | C[Si](C)(C)OC[C@@H](O)[C@@H](O[Si](C)(C)C)[C@H](O[Si](C)(C)C)[C@H](CO)O[Si](C)(C)C | 1865.0 | Semi standard non polar | 33892256 | | Sorbitol,4TMS,isomer #13 | C[Si](C)(C)OC[C@@H](O[Si](C)(C)C)[C@@H](O)[C@H](O[Si](C)(C)C)[C@H](CO)O[Si](C)(C)C | 1875.4 | Semi standard non polar | 33892256 | | Sorbitol,4TMS,isomer #14 | C[Si](C)(C)OC[C@@H](O[Si](C)(C)C)[C@@H](O[Si](C)(C)C)[C@H](O)[C@H](CO)O[Si](C)(C)C | 1872.6 | Semi standard non polar | 33892256 | | Sorbitol,4TMS,isomer #15 | C[Si](C)(C)OC[C@@H](O[Si](C)(C)C)[C@@H](O[Si](C)(C)C)[C@H](O[Si](C)(C)C)[C@@H](O)CO | 1880.0 | Semi standard non polar | 33892256 | | Sorbitol,4TMS,isomer #2 | C[Si](C)(C)OC[C@H](O[Si](C)(C)C)[C@@H](O[Si](C)(C)C)[C@H](O)[C@@H](CO)O[Si](C)(C)C | 1872.6 | Semi standard non polar | 33892256 | | Sorbitol,4TMS,isomer #3 | C[Si](C)(C)OC[C@H](O[Si](C)(C)C)[C@@H](O[Si](C)(C)C)[C@H](O)[C@H](O)CO[Si](C)(C)C | 1894.2 | Semi standard non polar | 33892256 | | Sorbitol,4TMS,isomer #4 | C[Si](C)(C)OC[C@H](O[Si](C)(C)C)[C@@H](O)[C@H](O[Si](C)(C)C)[C@@H](CO)O[Si](C)(C)C | 1875.4 | Semi standard non polar | 33892256 | | Sorbitol,4TMS,isomer #5 | C[Si](C)(C)OC[C@H](O[Si](C)(C)C)[C@@H](O)[C@H](O[Si](C)(C)C)[C@H](O)CO[Si](C)(C)C | 1890.0 | Semi standard non polar | 33892256 | | Sorbitol,4TMS,isomer #6 | C[Si](C)(C)OC[C@H](O[Si](C)(C)C)[C@@H](O)[C@H](O)[C@@H](CO[Si](C)(C)C)O[Si](C)(C)C | 1903.4 | Semi standard non polar | 33892256 | | Sorbitol,4TMS,isomer #7 | C[Si](C)(C)OC[C@H](O)[C@@H](O[Si](C)(C)C)[C@H](O[Si](C)(C)C)[C@@H](CO)O[Si](C)(C)C | 1865.0 | Semi standard non polar | 33892256 | | Sorbitol,4TMS,isomer #8 | C[Si](C)(C)OC[C@H](O)[C@@H](O[Si](C)(C)C)[C@H](O[Si](C)(C)C)[C@H](O)CO[Si](C)(C)C | 1880.4 | Semi standard non polar | 33892256 | | Sorbitol,4TMS,isomer #9 | C[Si](C)(C)OC[C@H](O)[C@@H](O[Si](C)(C)C)[C@H](O)[C@@H](CO[Si](C)(C)C)O[Si](C)(C)C | 1890.0 | Semi standard non polar | 33892256 | | Sorbitol,5TMS,isomer #1 | C[Si](C)(C)OC[C@H](O[Si](C)(C)C)[C@@H](O[Si](C)(C)C)[C@H](O[Si](C)(C)C)[C@@H](CO)O[Si](C)(C)C | 1905.0 | Semi standard non polar | 33892256 | | Sorbitol,5TMS,isomer #2 | C[Si](C)(C)OC[C@H](O[Si](C)(C)C)[C@@H](O[Si](C)(C)C)[C@H](O[Si](C)(C)C)[C@H](O)CO[Si](C)(C)C | 1877.2 | Semi standard non polar | 33892256 | | Sorbitol,5TMS,isomer #3 | C[Si](C)(C)OC[C@H](O[Si](C)(C)C)[C@@H](O[Si](C)(C)C)[C@H](O)[C@@H](CO[Si](C)(C)C)O[Si](C)(C)C | 1895.0 | Semi standard non polar | 33892256 | | Sorbitol,5TMS,isomer #4 | C[Si](C)(C)OC[C@H](O[Si](C)(C)C)[C@@H](O)[C@H](O[Si](C)(C)C)[C@@H](CO[Si](C)(C)C)O[Si](C)(C)C | 1895.0 | Semi standard non polar | 33892256 | | Sorbitol,5TMS,isomer #5 | C[Si](C)(C)OC[C@H](O)[C@@H](O[Si](C)(C)C)[C@H](O[Si](C)(C)C)[C@@H](CO[Si](C)(C)C)O[Si](C)(C)C | 1877.2 | Semi standard non polar | 33892256 | | Sorbitol,5TMS,isomer #6 | C[Si](C)(C)OC[C@@H](O[Si](C)(C)C)[C@@H](O[Si](C)(C)C)[C@H](O[Si](C)(C)C)[C@H](CO)O[Si](C)(C)C | 1905.0 | Semi standard non polar | 33892256 | | Sorbitol,6TMS,isomer #1 | C[Si](C)(C)OC[C@H](O[Si](C)(C)C)[C@@H](O[Si](C)(C)C)[C@H](O[Si](C)(C)C)[C@@H](CO[Si](C)(C)C)O[Si](C)(C)C | 1979.4 | Semi standard non polar | 33892256 | | Sorbitol,1TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO | 2030.5 | Semi standard non polar | 33892256 | | Sorbitol,1TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)O[C@@H](CO)[C@@H](O)[C@H](O)[C@H](O)CO | 1981.0 | Semi standard non polar | 33892256 | | Sorbitol,1TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)O[C@H]([C@@H](O)CO)[C@H](O)[C@H](O)CO | 1960.1 | Semi standard non polar | 33892256 | | Sorbitol,1TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)O[C@H]([C@H](O)CO)[C@H](O)[C@@H](O)CO | 1960.1 | Semi standard non polar | 33892256 | | Sorbitol,1TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)O[C@H](CO)[C@@H](O)[C@H](O)[C@@H](O)CO | 1981.0 | Semi standard non polar | 33892256 | | Sorbitol,1TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)CO | 2030.5 | Semi standard non polar | 33892256 | | Sorbitol,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](O)[C@H](O)[C@H](O)CO | 2252.8 | Semi standard non polar | 33892256 | | Sorbitol,2TBDMS,isomer #10 | CC(C)(C)[Si](C)(C)O[C@H]([C@@H](O)CO)[C@H](O[Si](C)(C)C(C)(C)C)[C@H](O)CO | 2225.9 | Semi standard non polar | 33892256 | | Sorbitol,2TBDMS,isomer #11 | CC(C)(C)[Si](C)(C)O[C@H](CO)[C@@H](O)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](O)CO | 2240.9 | Semi standard non polar | 33892256 | | Sorbitol,2TBDMS,isomer #12 | CC(C)(C)[Si](C)(C)OC[C@@H](O)[C@@H](O)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](O)CO | 2249.2 | Semi standard non polar | 33892256 | | Sorbitol,2TBDMS,isomer #13 | CC(C)(C)[Si](C)(C)O[C@H]([C@@H](CO)O[Si](C)(C)C(C)(C)C)[C@H](O)[C@@H](O)CO | 2239.9 | Semi standard non polar | 33892256 | | Sorbitol,2TBDMS,isomer #14 | CC(C)(C)[Si](C)(C)OC[C@@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O)[C@@H](O)CO | 2239.5 | Semi standard non polar | 33892256 | | Sorbitol,2TBDMS,isomer #15 | CC(C)(C)[Si](C)(C)OC[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H](O)[C@H](O)[C@@H](O)CO | 2252.8 | Semi standard non polar | 33892256 | | Sorbitol,2TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OC[C@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O)[C@H](O)CO | 2239.5 | Semi standard non polar | 33892256 | | Sorbitol,2TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC[C@H](O)[C@@H](O)[C@H](O[Si](C)(C)C(C)(C)C)[C@H](O)CO | 2249.2 | Semi standard non polar | 33892256 | | Sorbitol,2TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)OC[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O[Si](C)(C)C(C)(C)C | 2274.2 | Semi standard non polar | 33892256 | | Sorbitol,2TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO[Si](C)(C)C(C)(C)C | 2274.6 | Semi standard non polar | 33892256 | | Sorbitol,2TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)O[C@@H](CO)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O)[C@H](O)CO | 2239.9 | Semi standard non polar | 33892256 | | Sorbitol,2TBDMS,isomer #7 | CC(C)(C)[Si](C)(C)O[C@@H](CO)[C@@H](O)[C@H](O[Si](C)(C)C(C)(C)C)[C@H](O)CO | 2240.9 | Semi standard non polar | 33892256 | | Sorbitol,2TBDMS,isomer #8 | CC(C)(C)[Si](C)(C)O[C@@H](CO)[C@@H](O)[C@H](O)[C@@H](CO)O[Si](C)(C)C(C)(C)C | 2257.3 | Semi standard non polar | 33892256 | | Sorbitol,2TBDMS,isomer #9 | CC(C)(C)[Si](C)(C)OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](CO)O[Si](C)(C)C(C)(C)C | 2274.2 | Semi standard non polar | 33892256 | | Sorbitol,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O)[C@H](O)CO | 2488.3 | Semi standard non polar | 33892256 | | Sorbitol,3TBDMS,isomer #10 | CC(C)(C)[Si](C)(C)OC[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 2524.3 | Semi standard non polar | 33892256 | | Sorbitol,3TBDMS,isomer #11 | CC(C)(C)[Si](C)(C)O[C@@H](CO)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O[Si](C)(C)C(C)(C)C)[C@H](O)CO | 2514.6 | Semi standard non polar | 33892256 | | Sorbitol,3TBDMS,isomer #12 | CC(C)(C)[Si](C)(C)O[C@@H](CO)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O)[C@@H](CO)O[Si](C)(C)C(C)(C)C | 2535.7 | Semi standard non polar | 33892256 | | Sorbitol,3TBDMS,isomer #13 | CC(C)(C)[Si](C)(C)OC[C@@H](O)[C@@H](O)[C@H](O[Si](C)(C)C(C)(C)C)[C@H](CO)O[Si](C)(C)C(C)(C)C | 2541.7 | Semi standard non polar | 33892256 | | Sorbitol,3TBDMS,isomer #14 | CC(C)(C)[Si](C)(C)O[C@@H](CO)[C@@H](O)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](CO)O[Si](C)(C)C(C)(C)C | 2535.7 | Semi standard non polar | 33892256 | | Sorbitol,3TBDMS,isomer #15 | CC(C)(C)[Si](C)(C)OC[C@@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O)[C@H](CO)O[Si](C)(C)C(C)(C)C | 2550.6 | Semi standard non polar | 33892256 | | Sorbitol,3TBDMS,isomer #16 | CC(C)(C)[Si](C)(C)OC[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H](O)[C@H](O)[C@H](CO)O[Si](C)(C)C(C)(C)C | 2537.9 | Semi standard non polar | 33892256 | | Sorbitol,3TBDMS,isomer #17 | CC(C)(C)[Si](C)(C)O[C@H](CO)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](O)CO | 2514.6 | Semi standard non polar | 33892256 | | Sorbitol,3TBDMS,isomer #18 | CC(C)(C)[Si](C)(C)OC[C@@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](O)CO | 2510.6 | Semi standard non polar | 33892256 | | Sorbitol,3TBDMS,isomer #19 | CC(C)(C)[Si](C)(C)OC[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H](O)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](O)CO | 2515.2 | Semi standard non polar | 33892256 | | Sorbitol,3TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OC[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](O)[C@H](O[Si](C)(C)C(C)(C)C)[C@H](O)CO | 2515.2 | Semi standard non polar | 33892256 | | Sorbitol,3TBDMS,isomer #20 | CC(C)(C)[Si](C)(C)OC[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O)[C@@H](O)CO | 2488.3 | Semi standard non polar | 33892256 | | Sorbitol,3TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](O)[C@H](O)[C@@H](CO)O[Si](C)(C)C(C)(C)C | 2537.9 | Semi standard non polar | 33892256 | | Sorbitol,3TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)OC[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](O)[C@H](O)[C@H](O)CO[Si](C)(C)C(C)(C)C | 2524.3 | Semi standard non polar | 33892256 | | Sorbitol,3TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)OC[C@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O[Si](C)(C)C(C)(C)C)[C@H](O)CO | 2510.6 | Semi standard non polar | 33892256 | | Sorbitol,3TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)OC[C@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O)[C@@H](CO)O[Si](C)(C)C(C)(C)C | 2550.6 | Semi standard non polar | 33892256 | | Sorbitol,3TBDMS,isomer #7 | CC(C)(C)[Si](C)(C)OC[C@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O)[C@H](O)CO[Si](C)(C)C(C)(C)C | 2534.5 | Semi standard non polar | 33892256 | | Sorbitol,3TBDMS,isomer #8 | CC(C)(C)[Si](C)(C)OC[C@H](O)[C@@H](O)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](CO)O[Si](C)(C)C(C)(C)C | 2541.7 | Semi standard non polar | 33892256 | | Sorbitol,3TBDMS,isomer #9 | CC(C)(C)[Si](C)(C)OC[C@H](O)[C@@H](O)[C@H](O[Si](C)(C)C(C)(C)C)[C@H](O)CO[Si](C)(C)C(C)(C)C | 2534.5 | Semi standard non polar | 33892256 | | Sorbitol,4TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O[Si](C)(C)C(C)(C)C)[C@H](O)CO | 2735.0 | Semi standard non polar | 33892256 | | Sorbitol,4TBDMS,isomer #10 | CC(C)(C)[Si](C)(C)OC[C@H](O)[C@@H](O)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](CO[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 2754.9 | Semi standard non polar | 33892256 | | Sorbitol,4TBDMS,isomer #11 | CC(C)(C)[Si](C)(C)O[C@@H](CO)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](CO)O[Si](C)(C)C(C)(C)C | 2743.5 | Semi standard non polar | 33892256 | | Sorbitol,4TBDMS,isomer #12 | CC(C)(C)[Si](C)(C)OC[C@@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O[Si](C)(C)C(C)(C)C)[C@H](CO)O[Si](C)(C)C(C)(C)C | 2755.4 | Semi standard non polar | 33892256 | | Sorbitol,4TBDMS,isomer #13 | CC(C)(C)[Si](C)(C)OC[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H](O)[C@H](O[Si](C)(C)C(C)(C)C)[C@H](CO)O[Si](C)(C)C(C)(C)C | 2759.0 | Semi standard non polar | 33892256 | | Sorbitol,4TBDMS,isomer #14 | CC(C)(C)[Si](C)(C)OC[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O)[C@H](CO)O[Si](C)(C)C(C)(C)C | 2760.7 | Semi standard non polar | 33892256 | | Sorbitol,4TBDMS,isomer #15 | CC(C)(C)[Si](C)(C)OC[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](O)CO | 2735.0 | Semi standard non polar | 33892256 | | Sorbitol,4TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OC[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O)[C@@H](CO)O[Si](C)(C)C(C)(C)C | 2760.7 | Semi standard non polar | 33892256 | | Sorbitol,4TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O)[C@H](O)CO[Si](C)(C)C(C)(C)C | 2754.9 | Semi standard non polar | 33892256 | | Sorbitol,4TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)OC[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](O)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](CO)O[Si](C)(C)C(C)(C)C | 2759.0 | Semi standard non polar | 33892256 | | Sorbitol,4TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)OC[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](O)[C@H](O[Si](C)(C)C(C)(C)C)[C@H](O)CO[Si](C)(C)C(C)(C)C | 2767.4 | Semi standard non polar | 33892256 | | Sorbitol,4TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)OC[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](O)[C@H](O)[C@@H](CO[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 2767.7 | Semi standard non polar | 33892256 | | Sorbitol,4TBDMS,isomer #7 | CC(C)(C)[Si](C)(C)OC[C@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](CO)O[Si](C)(C)C(C)(C)C | 2755.4 | Semi standard non polar | 33892256 | | Sorbitol,4TBDMS,isomer #8 | CC(C)(C)[Si](C)(C)OC[C@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O[Si](C)(C)C(C)(C)C)[C@H](O)CO[Si](C)(C)C(C)(C)C | 2756.0 | Semi standard non polar | 33892256 | | Sorbitol,4TBDMS,isomer #9 | CC(C)(C)[Si](C)(C)OC[C@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O)[C@@H](CO[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 2767.4 | Semi standard non polar | 33892256 | | Sorbitol,5TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](CO)O[Si](C)(C)C(C)(C)C | 2965.2 | Semi standard non polar | 33892256 | | Sorbitol,5TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OC[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O[Si](C)(C)C(C)(C)C)[C@H](O)CO[Si](C)(C)C(C)(C)C | 2966.5 | Semi standard non polar | 33892256 | | Sorbitol,5TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O)[C@@H](CO[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 2957.9 | Semi standard non polar | 33892256 | | Sorbitol,5TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)OC[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](O)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](CO[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 2957.9 | Semi standard non polar | 33892256 | | Sorbitol,5TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)OC[C@H](O)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](CO[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 2966.5 | Semi standard non polar | 33892256 | | Sorbitol,5TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)OC[C@@H](O[Si](C)(C)C(C)(C)C)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O[Si](C)(C)C(C)(C)C)[C@H](CO)O[Si](C)(C)C(C)(C)C | 2965.2 | Semi standard non polar | 33892256 | | Sorbitol,6TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](O[Si](C)(C)C(C)(C)C)[C@H](O[Si](C)(C)C(C)(C)C)[C@@H](CO[Si](C)(C)C(C)(C)C)O[Si](C)(C)C(C)(C)C | 3191.2 | Semi standard non polar | 33892256 |
|
|---|
| Spectra |
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental GC-MS | GC-MS Spectrum - Sorbitol GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (Non-derivatized) | splash10-0ldj-0941000000-19d96a9ba7ba0c482c83 | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Sorbitol GC-MS (6 TMS) | splash10-014i-1973000000-d907b75f68a7927501a7 | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Sorbitol GC-EI-TOF (Non-derivatized) | splash10-0ldj-0941000000-19d96a9ba7ba0c482c83 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Sorbitol GC-MS (Non-derivatized) | splash10-014i-1973000000-d907b75f68a7927501a7 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Sorbitol GC-EI-TOF (Non-derivatized) | splash10-0ktb-0931000000-4ffadb4b25e8e2510d93 | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Sorbitol GC-MS (Non-derivatized) - 70eV, Positive | splash10-03k9-9500000000-97c5853d3e9b96f9d054 | 2016-09-22 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Sorbitol GC-MS (6 TMS) - 70eV, Positive | splash10-0a6s-7141193000-dcd9245e12314ee0ced4 | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Sorbitol GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Sorbitol GC-MS (TMS_1_1) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Sorbitol GC-MS (TMS_1_2) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Sorbitol GC-MS (TMS_1_3) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Sorbitol GC-MS (TMS_1_4) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Sorbitol GC-MS (TMS_1_5) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Sorbitol GC-MS (TMS_1_6) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Sorbitol GC-MS (TMS_2_1) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Sorbitol GC-MS (TMS_2_2) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Sorbitol GC-MS (TMS_2_3) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Sorbitol GC-MS (TMS_2_4) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Sorbitol GC-MS (TMS_2_5) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Sorbitol GC-MS (TMS_2_6) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Sorbitol GC-MS (TMS_2_7) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Sorbitol GC-MS (TMS_2_8) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Sorbitol GC-MS (TMS_2_9) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Sorbitol GC-MS (TMS_2_10) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Sorbitol GC-MS (TMS_2_11) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental LC-MS/MS | LC-MS/MS Spectrum - Sorbitol Quattro_QQQ 10V, Positive-QTOF (Annotated) | splash10-017j-8900000000-700285f86eac0a3501d1 | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Sorbitol Quattro_QQQ 25V, Positive-QTOF (Annotated) | splash10-066r-9000000000-84676b839b145250d67d | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Sorbitol Quattro_QQQ 40V, Positive-QTOF (Annotated) | splash10-052f-9000000000-dce72cab270c488d65da | 2012-07-24 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Sorbitol Linear Ion Trap , negative-QTOF | splash10-0ik9-2900000000-26a16e983791be7818f2 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Sorbitol Linear Ion Trap , negative-QTOF | splash10-0ik9-2900000000-6513771e890cbff9c402 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Sorbitol LC-ESI-ITFT , positive-QTOF | splash10-014j-0900000000-58e6917626e28d1830a2 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Sorbitol Linear Ion Trap , positive-QTOF | splash10-001i-0900000000-83169b2192f847cc0636 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Sorbitol Linear Ion Trap , positive-QTOF | splash10-03di-0900000000-4cd721d87e359527adf3 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Sorbitol Linear Ion Trap , positive-QTOF | splash10-03di-0900000000-b44f68c64bc74e79e9fc | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Sorbitol Linear Ion Trap , positive-QTOF | splash10-001i-0900000000-bbb5e957a9e67ddc411e | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Sorbitol Linear Ion Trap , positive-QTOF | splash10-000i-0900000000-772c3052966c05f29842 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Sorbitol Linear Ion Trap , positive-QTOF | splash10-000i-0900000000-d1fbea514e7f1c1628f2 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Sorbitol Linear Ion Trap , positive-QTOF | splash10-0udi-0790000000-b5b098cd5b4b4ec10821 | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Sorbitol Linear Ion Trap , positive-QTOF | splash10-0udi-0690000000-41d4e48d49836c63485c | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Sorbitol 35V, Negative-QTOF | splash10-0002-9200000000-9b9f891e480758a7d333 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Sorbitol 20V, Positive-QTOF | splash10-014j-0900000000-6ed9009701977f0a1c00 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Sorbitol 20V, Positive-QTOF | splash10-00kb-1900000000-7cc0793661b053aaf61e | 2021-09-20 | HMDB team, MONA | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Sorbitol 10V, Positive-QTOF | splash10-001i-2900000000-ef92b9091ecf5d15cdee | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Sorbitol 20V, Positive-QTOF | splash10-03di-9300000000-47f19bd4e51bff726536 | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Sorbitol 40V, Positive-QTOF | splash10-03dl-9100000000-60d5147d8e349337fe97 | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Sorbitol 10V, Negative-QTOF | splash10-07ju-8900000000-36f0fe0f346a6424162a | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Sorbitol 20V, Negative-QTOF | splash10-0c0c-9200000000-36e85b5ec098a45204c3 | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Sorbitol 40V, Negative-QTOF | splash10-0btc-9000000000-77703ee971336704163f | 2016-09-12 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Sorbitol 10V, Positive-QTOF | splash10-0002-3900000000-6801fbed6b67e0df2d20 | 2021-09-24 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Sorbitol 20V, Positive-QTOF | splash10-03dl-9000000000-4454dcd4b817c183328b | 2021-09-24 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-16 | Wishart Lab | View Spectrum | | Experimental 1D NMR | 13C NMR Spectrum (1D, 400 MHz, H2O, experimental) | 2021-10-10 | Wishart Lab | View Spectrum | | Experimental 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | 2012-12-05 | Wishart Lab | View Spectrum |
IR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M-H]-) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+H]+) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+Na]+) | 2023-02-03 | FELIX lab | View Spectrum |
|
|---|
| Biological Properties |
|---|
| Cellular Locations | |
|---|
| Biospecimen Locations | - Blood
- Cerebrospinal Fluid (CSF)
- Feces
- Saliva
- Urine
|
|---|
| Tissue Locations | - Adipose Tissue
- Adrenal Medulla
- Bladder
- Erythrocyte
- Eye Lens
- Fibroblasts
- Kidney
- Liver
- Neuron
- Placenta
- Platelet
- Prostate
- Retina
|
|---|
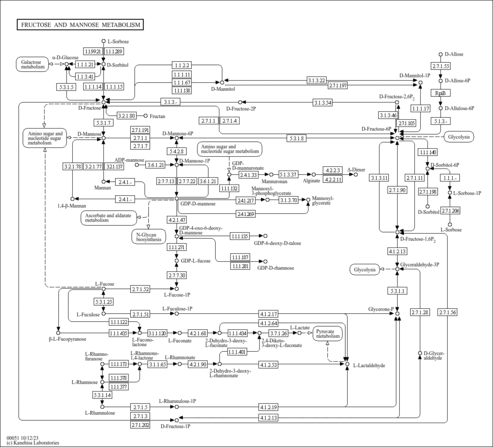
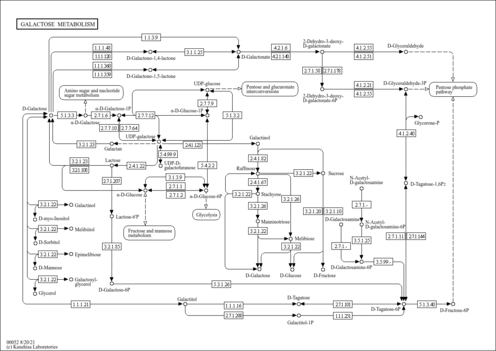
| Pathways | |
|---|
| Normal Concentrations |
|---|
| |
| Blood | Detected and Quantified | 1.09 +/- 0.37 uM | Adult (>18 years old) | Both | Normal | | details | | Blood | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Blood | Detected and Quantified | <2.198 uM | Adult (>18 years old) | Both | Normal | | details | | Blood | Detected and Quantified | 13.0 (4.0-24.0) uM | Adult (>18 years old) | Both | Normal | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 13.0 (9.49-16.5) uM | Adult (>18 years old) | Both | Normal | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 19.43 +/- 5.65 uM | Adult (>18 years old) | Not Specified | Normal | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 19.4 +/- 5.6 uM | Adult (>18 years old) | Both | Normal | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 15.6 +/- 1.9 uM | Adult (>18 years old) | Both | Normal | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 17.2 +/- 4.6 uM | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Infant (0-1 year old) | Both | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Children (6 - 18 years old) | Not Specified | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Not Specified | Not Specified | Normal | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Saliva | Detected and Quantified | 196.17 +/- 346.21 uM | Adult (>18 years old) | Both | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Not Specified | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Male | Normal | | details | | Saliva | Detected and Quantified | <1.00 uM | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected and Quantified | 3.9 (1.9-5.1) umol/mmol creatinine | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected and Quantified | 9.9 (2.5-18.7) umol/mmol creatinine | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected and Quantified | 3.5 +/- 2.24 umol/mmol creatinine | Adult (>18 years old) | Both | Normal | | details | | Urine | Detected and Quantified | 46.064 +/- 38.805 umol/mmol creatinine | Children (1 - 13 years old) | Not Specified | Normal | | details | | Urine | Detected and Quantified | 9.74 +/- 10.36 umol/mmol creatinine | Infant (0-1 year old) | Both | Normal | | details |
|
|---|
| Abnormal Concentrations |
|---|
| |
| Blood | Detected and Quantified | 17.0330 +/- 11.538 uM | Adult (>18 years old) | Both | Uremia | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 23.3 +/- 3.3 uM | Adult (>18 years old) | Both | Alzheimer's disease | | details | | Cerebrospinal Fluid (CSF) | Detected and Quantified | 22.89 +/- 3.34 uM | Not Specified | Not Specified | Alzheimer's disease | | details | | Feces | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Colorectal cancer | | details | | Feces | Detected but not Quantified | Not Quantified | Children (6 - 18 years old) | Not Specified | Crohns disease | | details | | Feces | Detected but not Quantified | Not Quantified | Children (6 - 18 years old) | Not Specified | Ulcerative colitis | | details | | Feces | Detected but not Quantified | Not Quantified | Children (6 - 18 years old) | Not Specified | Unclassified IBD | | details | | Urine | Detected but not Quantified | Not Quantified | Adult (>18 years old) | Both | Bladder cancer | | details | | Urine | Detected and Quantified | 52.238 umol/mmol creatinine | Children (1 - 13 years old) | Not Specified | Eosinophilic esophagitis | | details |
|
|---|
| Associated Disorders and Diseases |
|---|
| Disease References | | Uremia |
|---|
- Vanholder R, De Smet R, Glorieux G, Argiles A, Baurmeister U, Brunet P, Clark W, Cohen G, De Deyn PP, Deppisch R, Descamps-Latscha B, Henle T, Jorres A, Lemke HD, Massy ZA, Passlick-Deetjen J, Rodriguez M, Stegmayr B, Stenvinkel P, Tetta C, Wanner C, Zidek W: Review on uremic toxins: classification, concentration, and interindividual variability. Kidney Int. 2003 May;63(5):1934-43. doi: 10.1046/j.1523-1755.2003.00924.x. [PubMed:12675874 ]
| | Alzheimer's disease |
|---|
- Shetty HU, Holloway HW, Schapiro MB: Cerebrospinal fluid and plasma distribution of myo-inositol and other polyols in Alzheimer disease. Clin Chem. 1996 Feb;42(2):298-302. [PubMed:8595727 ]
| | Crohn's disease |
|---|
- Kolho KL, Pessia A, Jaakkola T, de Vos WM, Velagapudi V: Faecal and Serum Metabolomics in Paediatric Inflammatory Bowel Disease. J Crohns Colitis. 2017 Mar 1;11(3):321-334. doi: 10.1093/ecco-jcc/jjw158. [PubMed:27609529 ]
| | Ulcerative colitis |
|---|
- Kolho KL, Pessia A, Jaakkola T, de Vos WM, Velagapudi V: Faecal and Serum Metabolomics in Paediatric Inflammatory Bowel Disease. J Crohns Colitis. 2017 Mar 1;11(3):321-334. doi: 10.1093/ecco-jcc/jjw158. [PubMed:27609529 ]
| | Colorectal cancer |
|---|
- Goedert JJ, Sampson JN, Moore SC, Xiao Q, Xiong X, Hayes RB, Ahn J, Shi J, Sinha R: Fecal metabolomics: assay performance and association with colorectal cancer. Carcinogenesis. 2014 Sep;35(9):2089-96. doi: 10.1093/carcin/bgu131. Epub 2014 Jul 18. [PubMed:25037050 ]
| | Eosinophilic esophagitis |
|---|
- Slae, M., Huynh, H., Wishart, D.S. (2014). Analysis of 30 normal pediatric urine samples via NMR spectroscopy (unpublished work). NA.
|
|
|---|
| Associated OMIM IDs | |
|---|
| External Links |
|---|
| DrugBank ID | DB01638 |
|---|
| Phenol Explorer Compound ID | Not Available |
|---|
| FooDB ID | FDB011676 |
|---|
| KNApSAcK ID | C00001173 |
|---|
| Chemspider ID | 5576 |
|---|
| KEGG Compound ID | C00794 |
|---|
| BioCyc ID | SORBITOL |
|---|
| BiGG ID | Not Available |
|---|
| Wikipedia Link | Sorbitol |
|---|
| METLIN ID | Not Available |
|---|
| PubChem Compound | 5780 |
|---|
| PDB ID | Not Available |
|---|
| ChEBI ID | 17924 |
|---|
| Food Biomarker Ontology | Not Available |
|---|
| VMH ID | SBT_D |
|---|
| MarkerDB ID | MDB00000120 |
|---|
| Good Scents ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Liu, Haichao; Luo, Chen. Method for manufacturing sorbitol and mannitol with cellulose. Faming Zhuanli Shenqing Gongkai Shuomingshu (2007), 8pp. |
|---|
| Material Safety Data Sheet (MSDS) | Download (PDF) |
|---|
| General References | - Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM: Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009 Feb 12;457(7231):910-4. doi: 10.1038/nature07762. [PubMed:19212411 ]
- Airey CM, Price DE, Kemp JV, Perkins CM, Wales JK: The effect of aldose reductase inhibition on erythrocyte polyols and galactitol accumulation in diabetic patients. Diabet Med. 1989 Dec;6(9):804-8. [PubMed:2533041 ]
- Shetty HU, Holloway HW, Rapoport SI: Capillary gas chromatography combined with ion trap detection for quantitative profiling of polyols in cerebrospinal fluid and plasma. Anal Biochem. 1995 Jan 1;224(1):279-85. [PubMed:7710082 ]
- Sundkvist G, Dahlin LB, Nilsson H, Eriksson KF, Lindgarde F, Rosen I, Lattimer SA, Sima AA, Sullivan K, Greene DA: Sorbitol and myo-inositol levels and morphology of sural nerve in relation to peripheral nerve function and clinical neuropathy in men with diabetic, impaired, and normal glucose tolerance. Diabet Med. 2000 Apr;17(4):259-68. [PubMed:10821291 ]
- Morenkova SA: [Comparative analysis of dependence of saliva sorbitol and fructosamine levels on blood glucose level in patients with diabetes]. Biomed Khim. 2004;50(6):612-4. [PubMed:15707277 ]
- Bareford D, Jennings PE, Stone PC, Baar S, Barnett AH, Stuart J: Effects of hyperglycaemia and sorbitol accumulation on erythrocyte deformability in diabetes mellitus. J Clin Pathol. 1986 Jul;39(7):722-7. [PubMed:3090107 ]
- Ciuchi E, Odetti P, Prando R: Relationship between glutathione and sorbitol concentrations in erythrocytes from diabetic patients. Metabolism. 1996 May;45(5):611-3. [PubMed:8622605 ]
- Kwang-Hyok S, Ui-Nam P, Sarkar C, Bhadra R: A sensitive assay of red blood cell sorbitol level by high performance liquid chromatography: potential for diagnostic evaluation of diabetes. Clin Chim Acta. 2005 Apr;354(1-2):41-7. Epub 2005 Jan 18. [PubMed:15748598 ]
- Nau R: Osmotherapy for elevated intracranial pressure: a critical reappraisal. Clin Pharmacokinet. 2000 Jan;38(1):23-40. [PubMed:10668857 ]
- Belanger DR, Tierney MG, Dickinson G: Effect of sodium polystyrene sulfonate on lithium bioavailability. Ann Emerg Med. 1992 Nov;21(11):1312-5. [PubMed:1416324 ]
- Ciuchi E, Odetti P, Prando R: The effect of acute glutathione treatment on sorbitol level in erythrocytes from diabetic patients. Diabetes Metab. 1997 Feb;23(1):58-60. [PubMed:9059767 ]
- Shinohara R, Ohta Y, Yamauchi M, Ishiguro I: Improved fluorometric enzymatic sorbitol assay in human blood. Clin Chim Acta. 1998 May 25;273(2):171-84. [PubMed:9657347 ]
- Peterson MJ, Page MG, Just LJ, Aldinger CE, Malone JI: Applicability of red blood cell sorbitol measurements to monitor the clinical activity of sorbinil. Metabolism. 1986 Apr;35(4 Suppl 1):93-5. [PubMed:3515121 ]
- Vertommen J, Rillaerts E, Gysels M, De Leeuw I: Erythrocyte sorbitol content in diabetic patients: relation to metabolic control. Diabete Metab. 1987 Jun;13(3):182-6. [PubMed:3301442 ]
- Cunningham JJ, Mearkle PL, Brown RG: Vitamin C: an aldose reductase inhibitor that normalizes erythrocyte sorbitol in insulin-dependent diabetes mellitus. J Am Coll Nutr. 1994 Aug;13(4):344-50. [PubMed:7963139 ]
- Kamon N, Mabuchi H, Takeda R, Terashima H: Effects of aldose reductase inhibitor (ONO-2235) on human erythrocyte sorbitol concentrations in 75 g oral glucose tolerance tests. Horm Metab Res. 1991 May;23(5):226-9. [PubMed:1908433 ]
- Sizeland PC, Chambers ST, Lever M, Bason LM, Robson RA: Short-term response of nonurea organic osmolytes in human kidney to a water load and water deprivation. Am J Physiol. 1995 Feb;268(2 Pt 2):F227-33. [PubMed:7864160 ]
- van Griensven JM, Jusko WJ, Lemkes HH, Kroon R, Verhorst CJ, Chiang ST, Cohen AF: Tolrestat pharmacokinetic and pharmacodynamic effects on red blood cell sorbitol levels in normal volunteers and in patients with insulin-dependent diabetes. Clin Pharmacol Ther. 1995 Dec;58(6):631-40. [PubMed:8529328 ]
- Regenold WT, Kling MA, Hauser P: Elevated sorbitol concentration in the cerebrospinal fluid of patients with mood disorders. Psychoneuroendocrinology. 2000 Aug;25(6):593-606. [PubMed:10840171 ]
- Gehring H, Hornberger C, Dibbelt L, Dorges V, Eichenauer R, Schmucker P: Detecting and quantifying absorbed irrigation fluid by measuring mannitol and sorbitol concentrations in serum samples, and by ethanol monitoring. BJU Int. 2002 Feb;89(3):202-7. [PubMed:11856099 ]
- Burggraaf J, Schoemaker RC, Lentjes EG, Cohen AF: Sorbitol as a marker for drug-induced decreases of variable duration in liver blood flow in healthy volunteers. Eur J Pharm Sci. 2000 Dec;12(2):133-9. [PubMed:11102741 ]
- Elshenawy S, Pinney SE, Stuart T, Doulias PT, Zura G, Parry S, Elovitz MA, Bennett MJ, Bansal A, Strauss JF 3rd, Ischiropoulos H, Simmons RA: The Metabolomic Signature of the Placenta in Spontaneous Preterm Birth. Int J Mol Sci. 2020 Feb 4;21(3). pii: ijms21031043. doi: 10.3390/ijms21031043. [PubMed:32033212 ]
|
|---|